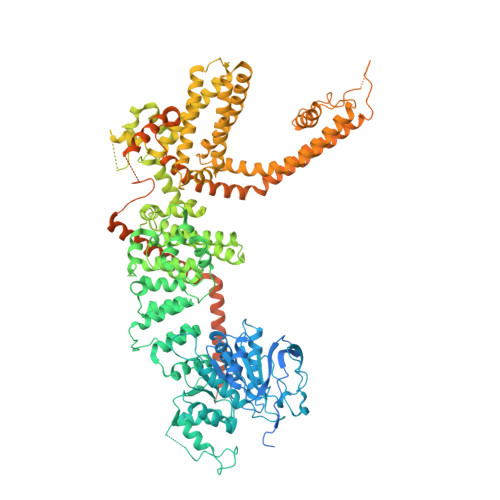
Structural and functional analyses of a GPCR-inhibited ion channel TRPM3.
Zhao, C., MacKinnon, R.(2023) Neuron 111: 81
- PubMed: 36283409
- DOI: https://doi.org/10.1016/j.neuron.2022.10.002
- Primary Citation of Related Structures:
8DDQ, 8DDR, 8DDS, 8DDT, 8DDU, 8DDV, 8DDW, 8DDX, 8ED7, 8ED8, 8ED9 - PubMed Abstract:
G-protein coupled receptors (GPCRs) govern the physiological response to stimuli by modulating the activity of downstream effectors, including ion channels. TRPM3 is an ion channel inhibited by GPCRs through direct interaction with G protein (Gβγ) released upon their activation. This GPCR-TRPM3 signaling pathway contributes to the analgesic effect of morphine. Here, we characterized Gβγ inhibition of TRPM3 using electrophysiology and single particle cryo-electron microscopy (cryo-EM). From electrophysiology, we obtained a half inhibition constant (IC50) of ∼240 nM. Using cryo-EM, we determined structures of mouse TRPM3 expressed in human cells with and without Gβγ and with and without PIP 2 , a lipid required for TRPM3 activity, at resolutions of 2.7-4.7 Å. Gβγ-TRPM3 interfaces vary depending on PIP 2 occupancy; however, in all cases, Gβγ appears loosely attached to TRPM3. The IC50 in electrophysiology experiments raises the possibility that additional unknown factors may stabilize the TRPM3-Gβγ complex.
- Laboratory of Molecular Neurobiology and Biophysics, Howard Hughes Medical Institute, the Rockefeller University, New York, NY 10065, United States.
Organizational Affiliation: