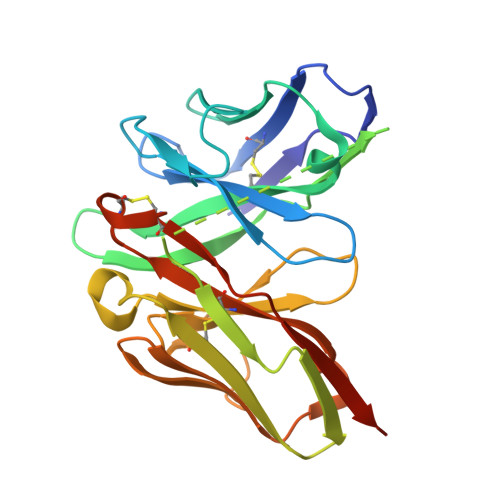
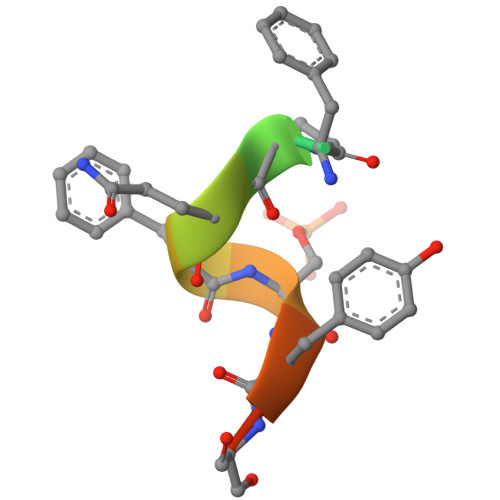
Origin of high binding affinity and selectivity of phosphorylated epitope-specific rabbit antibodies
Kasahara, K., Kawade, R., Nakakido, M., Matsunaga, R., Akiba, H., Entzminger, K.C., Maruyama, T., Okumura, S.C.J., Caaveiro, J.M.M., Kuroda, D., Tsumoto, K.To be published.