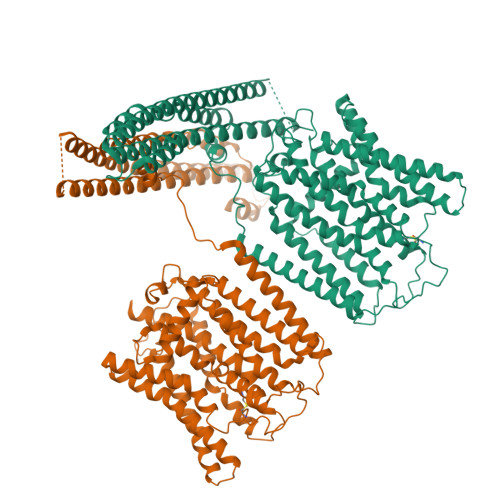
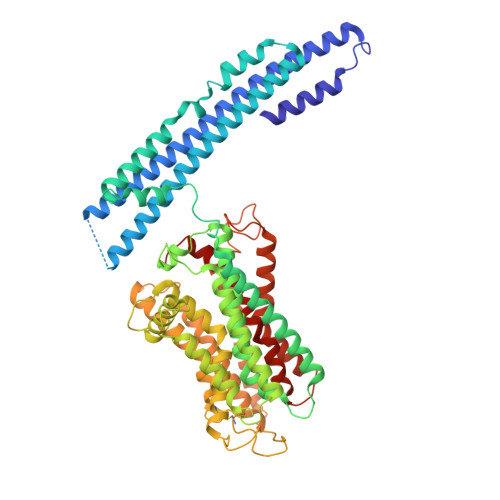
Structural basis for inositol pyrophosphate gating of the phosphate channel XPR1.
Lu, Y., Yue, C.X., Zhang, L., Yao, D., Xia, Y., Zhang, Q., Zhang, X., Li, S., Shen, Y., Cao, M., Guo, C.R., Qin, A., Zhao, J., Zhou, L., Yu, Y., Cao, Y.(2024) Science 386: eadp3252-eadp3252
- PubMed: 39325866
- DOI: https://doi.org/10.1126/science.adp3252
- Primary Citation of Related Structures:
8YET, 8YEX, 8YF4, 8YFD, 8YFU, 8YFW, 8YFX, 9IWS - PubMed Abstract:
Precise regulation of intracellular phosphate (Pi) is critical for cellular function, with XPR1 serving as the sole Pi exporter in humans. The mechanism of Pi efflux, activated by inositol pyrophosphates (PP-IPs), has remained unclear. This study presents cryo-electron microscopy structures of XPR1 in multiple conformations, revealing a transmembrane pathway for Pi export and a dual-binding activation pattern by PP-IPs. A canonical binding site is located at the dimeric interface of SPX domains, and a second site, biased toward PP-IPs, is found between the transmembrane and SPX domains. By integrating structural studies with electrophysiological analyses, we characterize XPR1 as an IPs/PP-IPs-activated phosphate channel. The interplay among its TMDs, SPX domains, and IPs/PP-IPs orchestrates the conformational transition between its closed and open states.
Organizational Affiliation:
Institute of Precision Medicine, the Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200125, China.