Structural insights into immune escape at killer T cell epitope by SARS-CoV-2 Spike Y453F variants.
Deng, S., Xu, Z., Wang, M., Hu, J., Liu, Z., Zhu, F., Zheng, P., Kombe Kombe, A.J., Zhang, H., Wu, S., Jin, T.(2024) J Biol Chem 300: 107563-107563
- PubMed: 39002680
- DOI: https://doi.org/10.1016/j.jbc.2024.107563
- Primary Citation of Related Structures:
8YE4, 8ZV9 - PubMed Abstract:
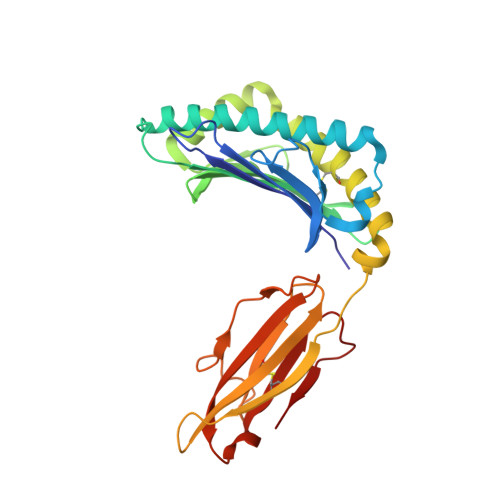
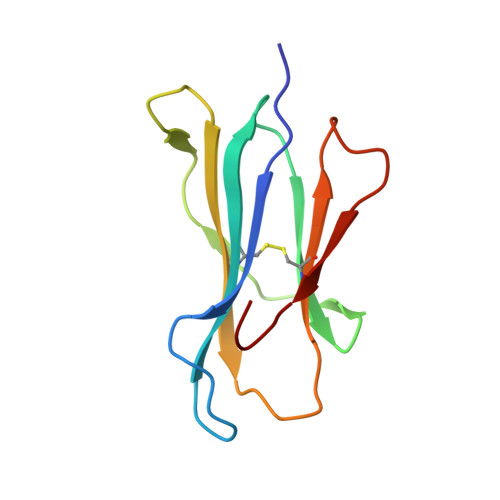
CD8 + T cell immunity, mediated by human leukocyte antigen (HLA) and T cell receptor (TCR), plays a critical role in conferring immune memory and protection against viral pathogens. The emergence of SARS-CoV-2 variants poses a serious challenge to the efficacy of current vaccines. Whereas numerous SARS-CoV-2 mutations associated with immune escape from CD8 + T cells have been documented, the molecular effects of most mutations on epitope-specific TCR recognition remain largely unexplored. Here, we studied an HLA-A24-restricted NYN epitope (Spike 448-456 ) that elicits broad CD8 + T cell responses in COVID-19 patients characterized by a common TCR repertoire. Four natural mutations, N450K, L452Q, L452R, and Y453F, arose within the NYN epitope and have been transmitted in certain viral lineages. Our findings indicate that these mutations have minimal impact on the epitope's presentation by cell surface HLA, yet they diminish the affinities of their respective peptide-HLA complexes (pHLAs) for NYN peptide-specific TCRs, particularly L452R and Y453F. Furthermore, we determined the crystal structure of HLA-A24 loaded with the Y453F peptide (NYNYLFRLF), and subsequently a ternary structure of the public TCR NYN-I complexed to the original NYN-HLA-A24 (NYNYLYRLF). Our structural analysis unveiled that despite competent presentation by HLA, the mutant Y453F peptide failed to establish a stable TCR-pHLA ternary complex due to reduced peptide: TCR contacts. This study supports the idea that cellular immunity restriction is an important driving force behind viral evolution.
Organizational Affiliation:
Department of Obstetrics and Gynecology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, Center for Advanced Interdisciplinary Science and Biomedicine of IHM, University of Science and Technology of China, Hefei, Anhui, P.R. China.