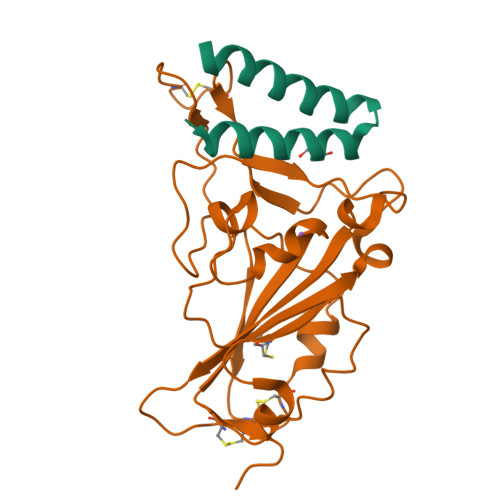
Development of a short-peptide inhibiting any SARS-CoV-2 variants based on structural biology
Nakamura, S., Tanimura, Y., Nomura, R., Suzuki, H., Nishikawa, K., Kamegawa, A., Numoto, N., Tanaka, A., Kawabata, S., Sakaguchi, S., Emi, A., Suzuki, Y., Fujiyoshi, Y.To be published.