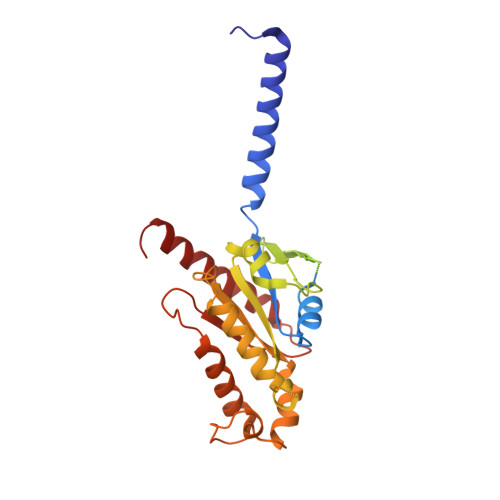
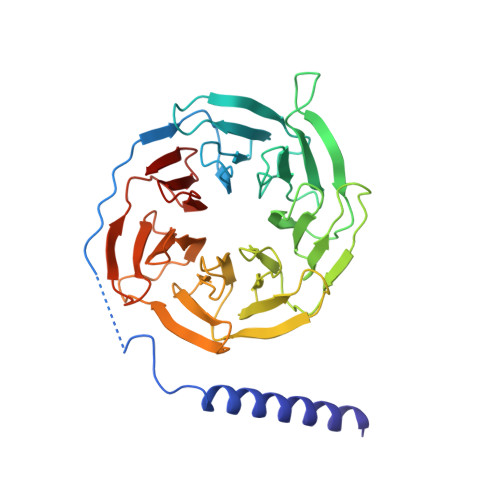
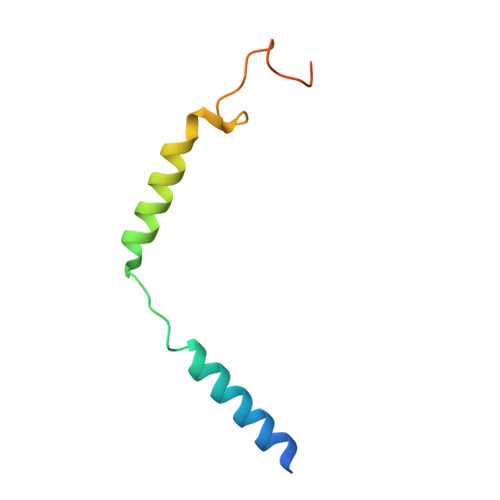
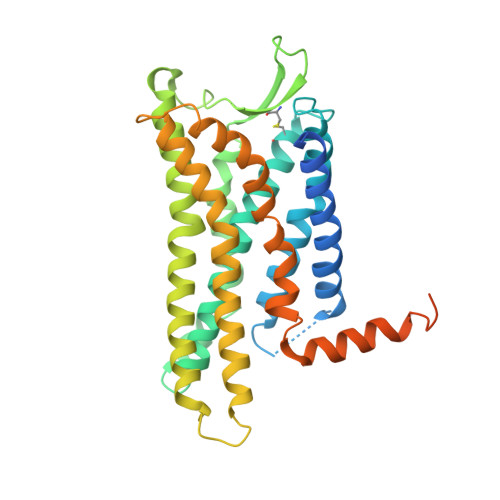
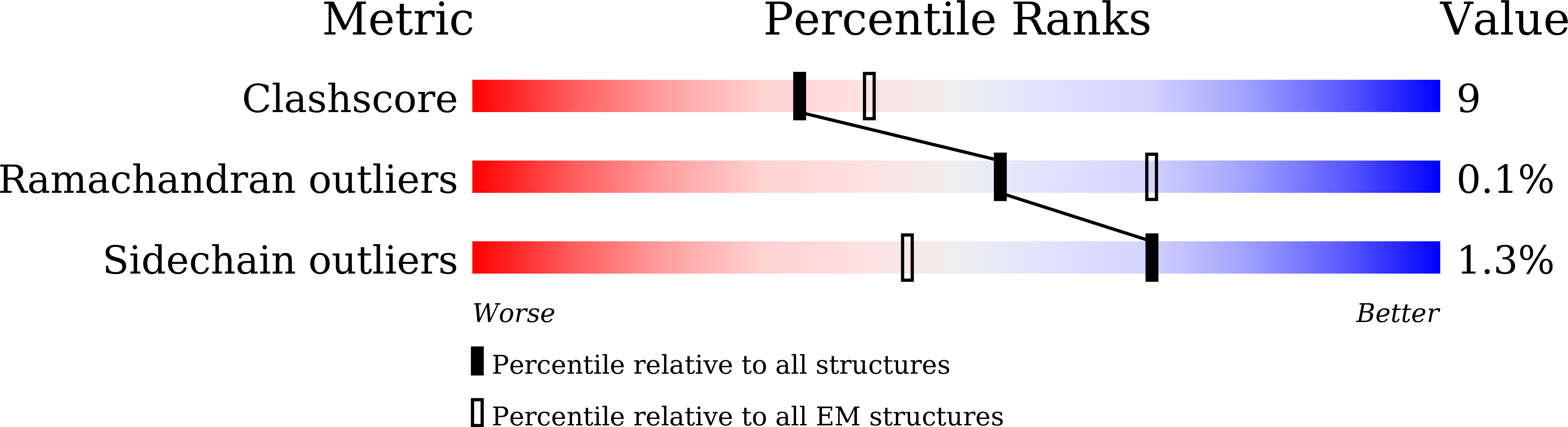Structure-based design of non-hypertrophic apelin receptor modulator.
Wang, W.W., Ji, S.Y., Zhang, W., Zhang, J., Cai, C., Hu, R., Zang, S.K., Miao, L., Xu, H., Chen, L.N., Yang, Z., Guo, J., Qin, J., Shen, D.D., Liang, P., Zhang, Y., Zhang, Y.(2024) Cell 187: 1460-1475.e20
- PubMed: 38428423
- DOI: https://doi.org/10.1016/j.cell.2024.02.004
- Primary Citation of Related Structures:
8XZF, 8XZG, 8XZH, 8XZI, 8XZJ - PubMed Abstract:
Apelin is a key hormone in cardiovascular homeostasis that activates the apelin receptor (APLNR), which is regarded as a promising therapeutic target for cardiovascular disease. However, adverse effects through the β-arrestin pathway limit its pharmacological use. Here, we report cryoelectron microscopy (cryo-EM) structures of APLNR-G i1 complexes bound to three agonists with divergent signaling profiles. Combined with functional assays, we have identified "twin hotspots" in APLNR as key determinants for signaling bias, guiding the rational design of two exclusive G-protein-biased agonists WN353 and WN561. Cryo-EM structures of WN353- and WN561-stimulated APLNR-G protein complexes further confirm that the designed ligands adopt the desired poses. Pathophysiological experiments have provided evidence that WN561 demonstrates superior therapeutic effects against cardiac hypertrophy and reduced adverse effects compared with the established APLNR agonists. In summary, our designed APLNR modulator may facilitate the development of next-generation cardiovascular medications.
Organizational Affiliation:
Department of Pharmacology and Department of Pathology of Sir Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310058, China; Liangzhu Laboratory, Zhejiang University, 1369 West Wenyi Road, Hangzhou 311121, China; Center for Structural Pharmacology and Therapeutics Development, Sir Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310016, China.