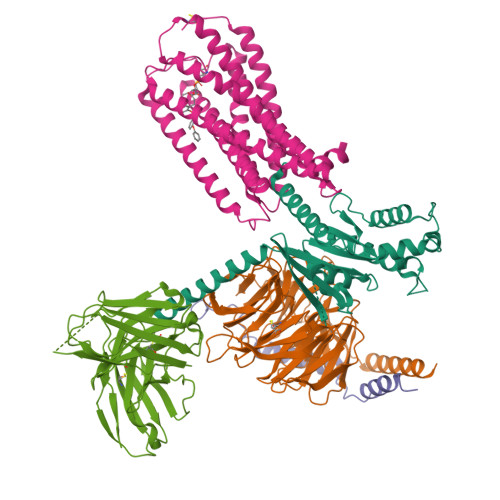
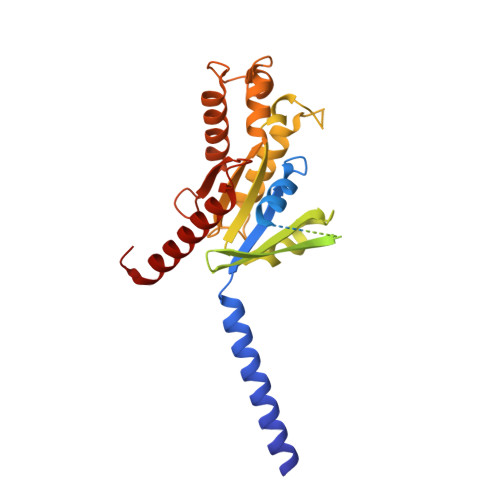
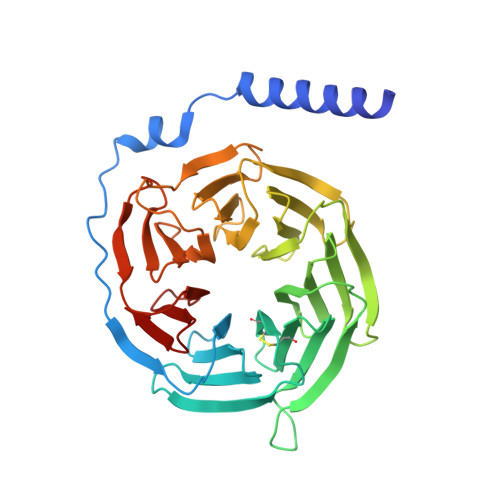
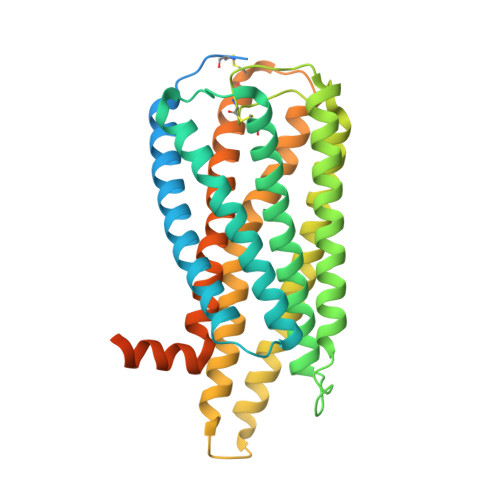
Structural basis for lysophosphatidylserine recognition by GPR34.
Izume, T., Kawahara, R., Uwamizu, A., Chen, L., Yaginuma, S., Omi, J., Kawana, H., Hou, F., Sano, F.K., Tanaka, T., Kobayashi, K., Okamoto, H.H., Kise, Y., Ohwada, T., Aoki, J., Shihoya, W., Nureki, O.(2024) Nat Commun 15: 902-902
- PubMed: 38326347
- DOI: https://doi.org/10.1038/s41467-024-45046-z
- Primary Citation of Related Structures:
8XBE, 8XBG, 8XBH, 8XBI - PubMed Abstract:
GPR34 is a recently identified G-protein coupled receptor, which has an immunomodulatory role and recognizes lysophosphatidylserine (LysoPS) as a putative ligand. Here, we report cryo-electron microscopy structures of human GPR34-G i complex bound with one of two ligands bound: either the LysoPS analogue S3E-LysoPS, or M1, a derivative of S3E-LysoPS in which oleic acid is substituted with a metabolically stable aromatic fatty acid surrogate. The ligand-binding pocket is laterally open toward the membrane, allowing lateral entry of lipidic agonists into the cavity. The amine and carboxylate groups of the serine moiety are recognized by the charged residue cluster. The acyl chain of S3E-LysoPS is bent and fits into the L-shaped hydrophobic pocket in TM4-5 gap, and the aromatic fatty acid surrogate of M1 fits more appropriately. Molecular dynamics simulations further account for the LysoPS-regioselectivity of GPR34. Thus, using a series of structural and physiological experiments, we provide evidence that chemically unstable 2-acyl LysoPS is the physiological ligand for GPR34. Overall, we anticipate the present structures will pave the way for development of novel anticancer drugs that specifically target GPR34.
Organizational Affiliation:
Department of Biological Sciences, Graduate School of Science, The University of Tokyo, Bunkyo-ku, Tokyo, 113-0033, Japan.