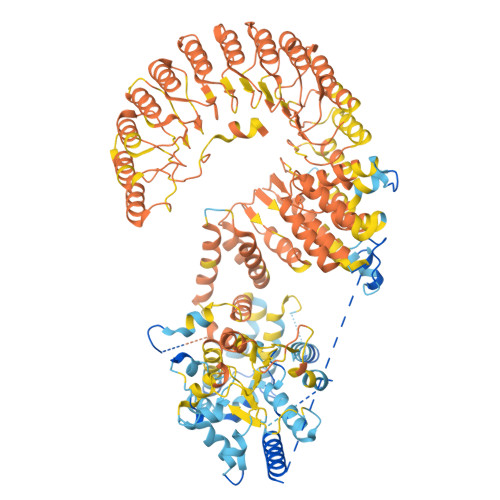
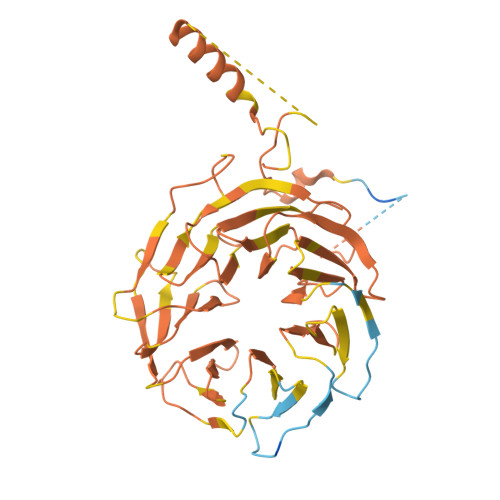
Cryo-EM structure of the human subcortical maternal complex and the associated discovery of infertility-associated variants.
Chi, P., Ou, G., Liu, S., Ma, Q., Lu, Y., Li, J., Li, J., Qi, Q., Han, Z., Zhang, Z., Liu, Q., Guo, L., Chen, J., Wang, X., Huang, W., Li, L., Deng, D.(2024) Nat Struct Mol Biol
- PubMed: 39379527
- DOI: https://doi.org/10.1038/s41594-024-01396-2
- Primary Citation of Related Structures:
8X7V, 8X7W - PubMed Abstract:
The functionally conserved subcortical maternal complex (SCMC) is essential for early embryonic development in mammals. Reproductive disorders caused by pathogenic variants in NLRP5, TLE6 and OOEP, three core components of the SCMC, have attracted much attention over the past several years. Evaluating the pathogenicity of a missense variant in the SCMC is limited by the lack of information on its structure, although we recently solved the structure of the mouse SCMC and proposed that reproductive disorders caused by pathogenic variants are related to the destabilization of the SCMC core complex. Here we report the cryogenic electron microscopy structure of the human SCMC and uncover that the pyrin domain of NLRP5 is essential for the stability of SCMC. By combining prediction of SCMC stability and in vitro reconstitution, we provide a method for identifying deleterious variants, and we successfully identify a new pathogenic variant of TLE6 (p.A396T). Thus, on the basis of the structure of the human SCMC, we offer a strategy for the diagnosis of reproductive disorders and the discovery of new infertility-associated variants.
Organizational Affiliation:
Department of Obstetrics and Gynecology, Key Laboratory of Birth Defects and Related Disease of Women and Children of MOE, State Key Laboratory of Biotherapy, West China Second University Hospital, Sichuan University, Chengdu, China.