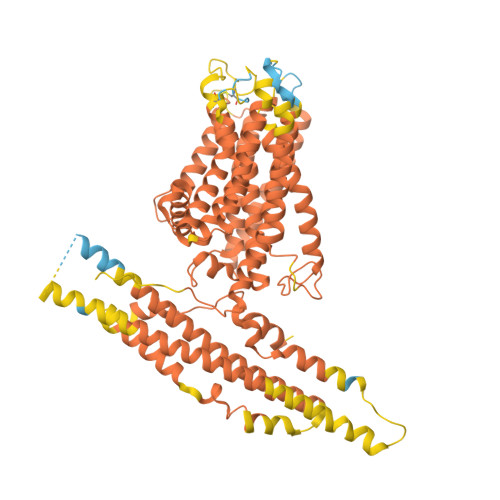
Human XPR1 structures reveal phosphate export mechanism.
Yan, R., Chen, H., Liu, C., Zhao, J., Wu, D., Jiang, J., Gong, J., Jiang, D.(2024) Nature
- PubMed: 39169184
- DOI: https://doi.org/10.1038/s41586-024-07852-9
- Primary Citation of Related Structures:
8X5B, 8X5E, 8X5F - PubMed Abstract:
Inorganic phosphate (Pi) is a fundamental macronutrient for all living organisms, the homeostasis of which is critical for numerous biological activities 1-3 . As the only known human Pi exporter to date, XPR1 has an indispensable role in cellular Pi homeostasis 4,5 . Dysfunction of XPR1 is associated with neurodegenerative disease 6-8 . However, the mechanisms underpinning XPR1-mediated Pi efflux and regulation by the intracellular inositol polyphosphate (InsPP) sensor SPX domain remain poorly understood. Here we present cryo-electron microscopy structures of human XPR1 in Pi-bound closed, open and InsP 6 -bound forms, revealing the structural basis for XPR1 gating and regulation by InsPPs. XPR1 consists of an N-terminal SPX domain, a dimer-formation core domain and a Pi transport domain. Within the transport domain, three basic clusters are responsible for Pi binding and transport, and a conserved W573 acts as a molecular switch for gating. In addition, the SPX domain binds to InsP 6 and facilitates Pi efflux by liberating the C-terminal loop that limits Pi entry. This study provides a conceptual framework for the mechanistic understanding of Pi homeostasis by XPR1 homologues in fungi, plants and animals.
Organizational Affiliation:
Laboratory of Soft Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing, China.