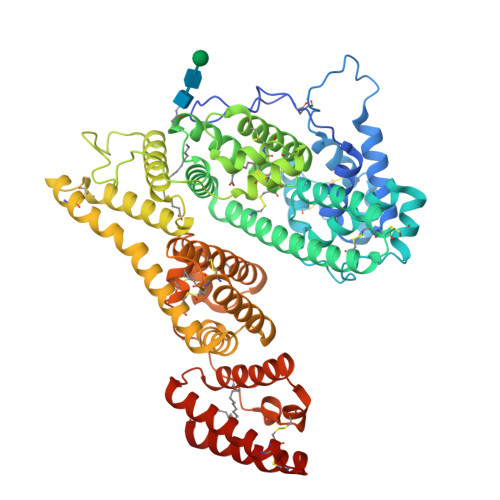
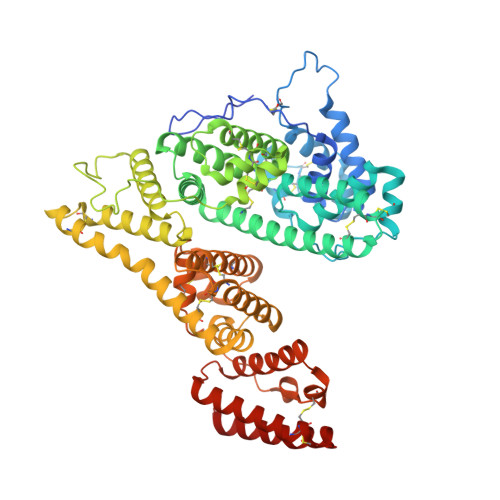
Structural characteristics of alpha-fetoprotein, including N-glycosylation, metal ion and fatty acid binding sites.
Liu, K., Wu, C., Zhu, M., Xu, J., Lin, B., Lin, H., Liu, Z., Li, M.(2024) Commun Biol 7: 505-505
- PubMed: 38678117
- DOI: https://doi.org/10.1038/s42003-024-06219-0
- Primary Citation of Related Structures:
8X1N - PubMed Abstract:
Alpha-fetoprotein (AFP), a serum glycoprotein, is expressed during embryonic development and the pathogenesis of liver cancer. It serves as a clinical tumor marker, function as a carcinogen, immune suppressor, and transport vehicle; but the detailed AFP structural information has not yet been reported. In this study, we used single-particle cryo-electron microscopy(cryo-EM) to analyze the structure of the recombinant AFP obtained a 3.31 Å cryo-EM structure and built an atomic model of AFP. We observed and identified certain structural features of AFP, including N-glycosylation at Asn251, four natural fatty acids bound to distinct domains, and the coordination of metal ions by residues His22, His264, His268, and Asp280. Furthermore, we compared the structural similarities and differences between AFP and human serum albumin. The elucidation of AFP's structural characteristics not only contributes to a deeper understanding of its functional mechanisms, but also provides a structural basis for developing AFP-based drug vehicles.
Organizational Affiliation:
Hainan Provincial Key Laboratory of Carcinogenesis and Intervention, Hainan Medical College, Haikou, 571199, Hainan, PR China.