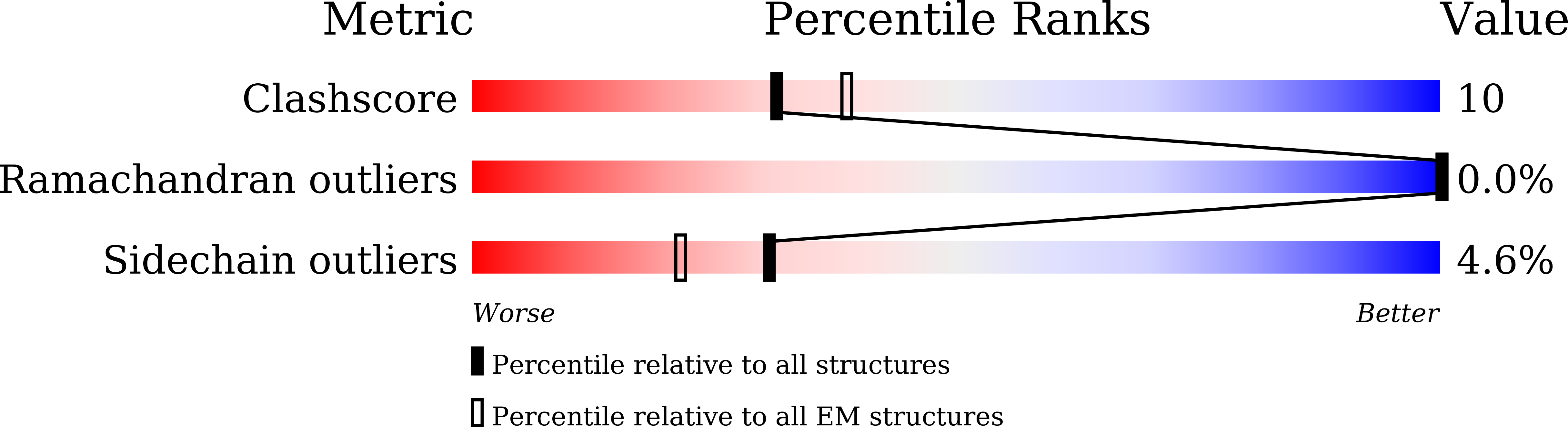Structural insights into thermophilic chaperonin complexes.
Liao, Z., Gopalasingam, C.C., Kameya, M., Gerle, C., Shigematsu, H., Ishii, M., Arakawa, T., Fushinobu, S.(2024) Structure 32: 679-689.e4
- PubMed: 38492570
- DOI: https://doi.org/10.1016/j.str.2024.02.012
- Primary Citation of Related Structures:
8WU4, 8WUC, 8WUW, 8WUX - PubMed Abstract:
Group I chaperonins are dual heptamer protein complexes that play significant roles in protein homeostasis. The structure and function of the Escherichia coli chaperonin are well characterized. However, the dynamic properties of chaperonins, such as large ATPase-dependent conformational changes by binding of lid-like co-chaperonin GroES, have made structural analyses challenging, and our understanding of these changes during the turnover of chaperonin complex formation is limited. In this study, we used single-particle cryogenic electron microscopy to investigate the structures of GroES-bound chaperonin complexes from the thermophilic hydrogen-oxidizing bacteria Hydrogenophilus thermoluteolus and Hydrogenobacter thermophilus in the presence of ATP and AMP-PNP. We captured the structure of an intermediate state chaperonin complex, designated as an asymmetric football-shaped complex, and performed analyses to decipher the dynamic structural variations. Our structural analyses of inter- and intra-subunit communications revealed a unique mechanism of complex formation through the binding of a second GroES to a bullet-shaped complex.
Organizational Affiliation:
Graduate School of Agricultural and Life Sciences, The University of Tokyo, Bunkyo City, Tokyo 113-8654, Japan.