Discovery of Novel 11-Membered Templates as Squalene Synthase Inhibitors.
Haginoya, N., Suzuki, M., Suzuki, M., Ishigai, Y., Terayama, K., Kanda, A., Sugita, K.(2024) J Med Chem 67: 5305-5314
- PubMed: 38517948
- DOI: https://doi.org/10.1021/acs.jmedchem.3c01500
- Primary Citation of Related Structures:
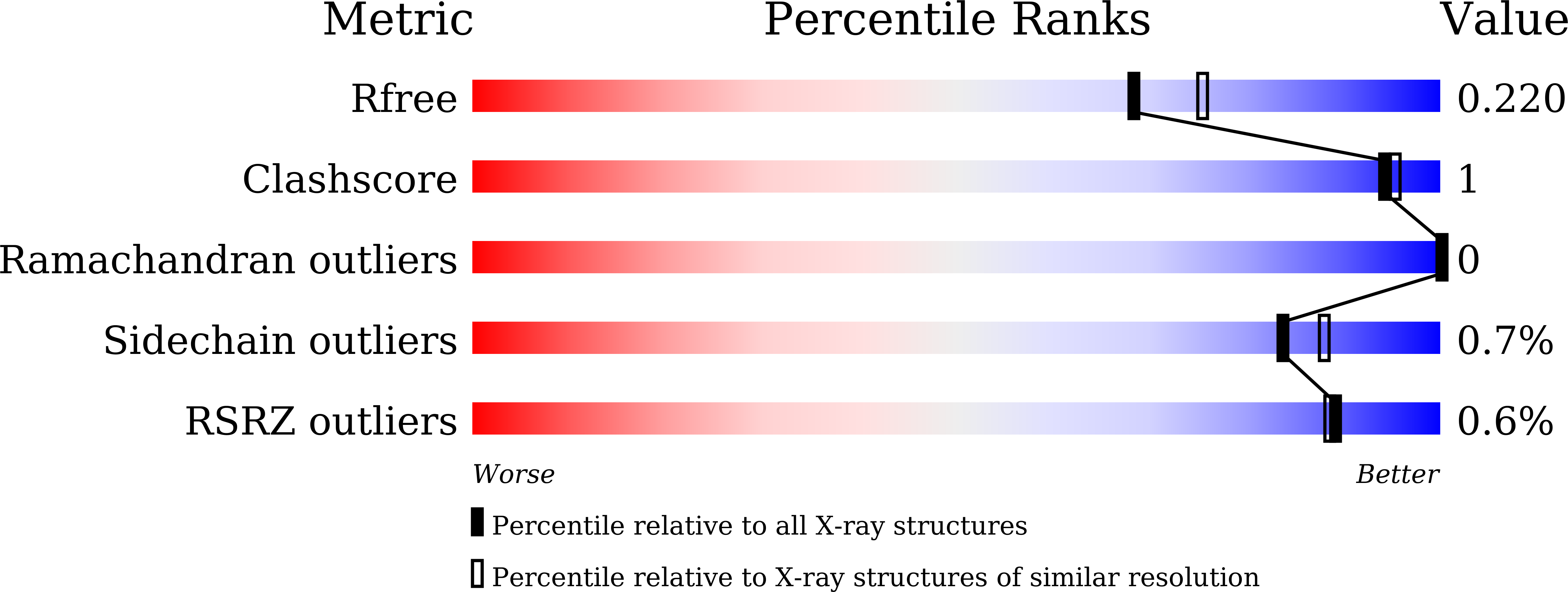
8WTQ, 8WTR - PubMed Abstract:
Squalene synthase is one of the most promising pharmaceutical targets to treat hyperlipidemia. Inhibition of the squalene synthase causes a decrease in the hepatic cholesterol concentration. We have already reported the design and synthesis of highly potent benzhydrol-type squalene inhibitors. Although these templates showed unique and potent cyclic active conformations via intramolecular hydrogen bonds, the in vivo cholesterol-lowering efficacy was insufficient. We attempted to improve their potential as an orally active medicine. In our medicinal chemistry effort, cyclized 11-membered ring templates were acquired. The novel series of compounds exhibited potent squalene synthase inhibitory activity, and one of the derivatives, isomer A -( 1 S , 3 R )- 14i , showed plasma lipid-lowering efficacy in hamster and marmoset repeated-dose studies. Our findings provide valuable insights into the design and development of novel and unique 11-membered ring-type highly potent squalene synthase inhibitors.
Organizational Affiliation:
Daiichi Sankyo RD Novare Co., Ltd., 1-16-13 Kita-Kasai, Edogawa-ku, 134-8630 Tokyo, Japan.