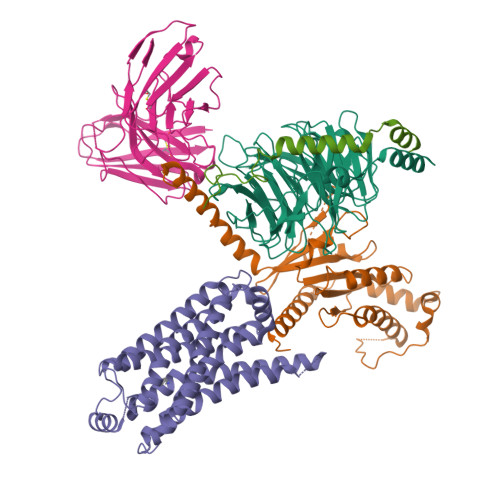
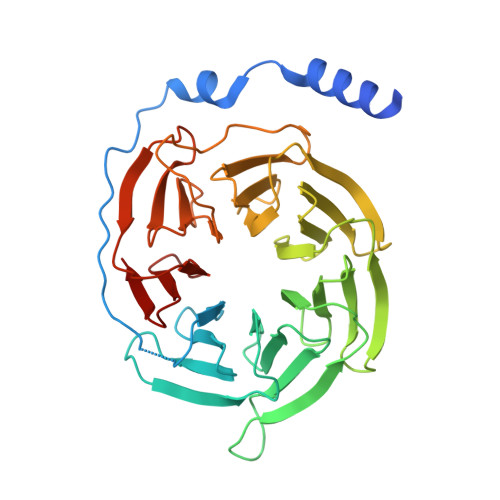
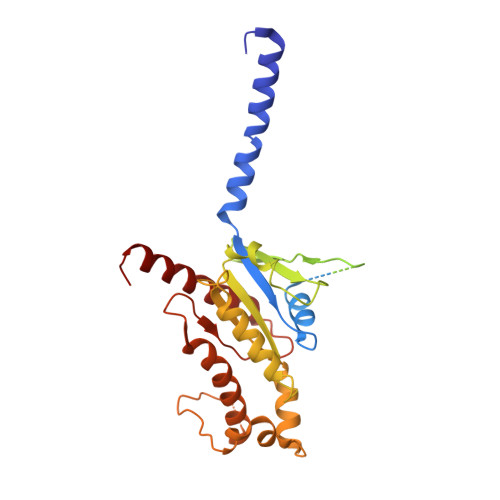
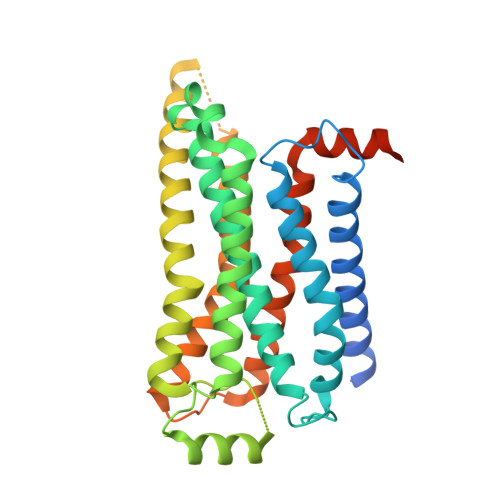
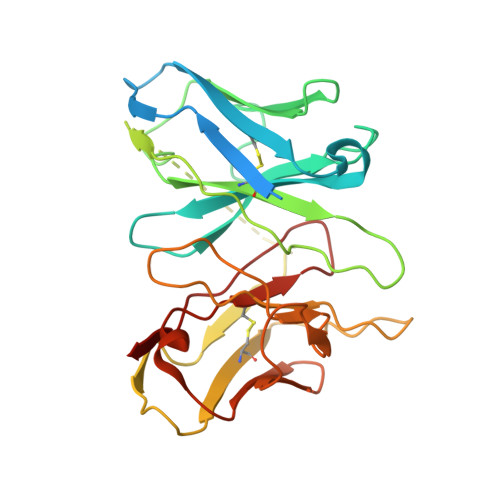
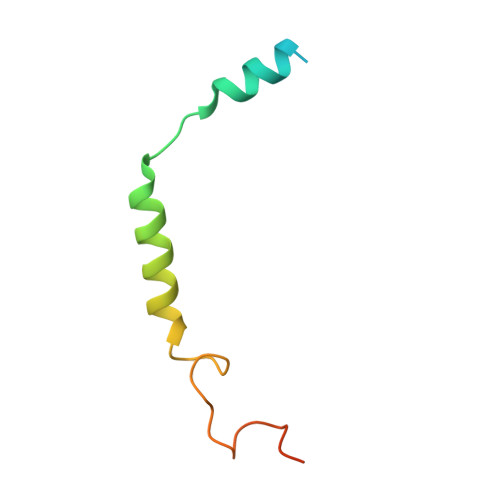
Structural and signaling mechanisms of TAAR1 enabled preferential agonist design.
Shang, P., Rong, N., Jiang, J.J., Cheng, J., Zhang, M.H., Kang, D., Qi, L., Guo, L., Yang, G.M., Liu, Q., Zhou, Z., Li, X.B., Zhu, K.K., Meng, Q.B., Han, X., Yan, W., Kong, Y., Yang, L., Wang, X., Lei, D., Feng, X., Liu, X., Yu, X., Wang, Y., Li, Q., Shao, Z.H., Yang, F., Sun, J.P.(2023) Cell 186: 5347-5362.e24
- PubMed: 37963465
- DOI: https://doi.org/10.1016/j.cell.2023.10.014
- Primary Citation of Related Structures:
8WC3, 8WC4, 8WC5, 8WC6, 8WC7, 8WC8, 8WC9, 8WCA, 8WCB, 8WCC - PubMed Abstract:
Trace amine-associated receptor 1 (TAAR1) senses a spectrum of endogenous amine-containing metabolites (EAMs) to mediate diverse psychological functions and is useful for schizophrenia treatment without the side effects of catalepsy. Here, we systematically profiled the signaling properties of TAAR1 activation and present nine structures of TAAR1-Gs/Gq in complex with EAMs, clinical drugs, and synthetic compounds. These structures not only revealed the primary amine recognition pocket (PARP) harboring the conserved acidic D 3.32 for conserved amine recognition and "twin" toggle switch for receptor activation but also elucidated that targeting specific residues in the second binding pocket (SBP) allowed modulation of signaling preference. In addition to traditional drug-induced Gs signaling, Gq activation by EAM or synthetic compounds is beneficial to schizophrenia treatment. Our results provided a structural and signaling framework for molecular recognition by TAAR1, which afforded structural templates and signal clues for TAAR1-targeted candidate compounds design.
Organizational Affiliation:
NHC Key Laboratory of Otorhinolaryngology, Qilu hospital and School of Basic Medical Sciences, Shandong University, Jinan, Shandong 250012, China; Advanced Medical Research Institute and Meili Lake Translational Research Park, Shandong University, Jinan, Shandong 250012, China.