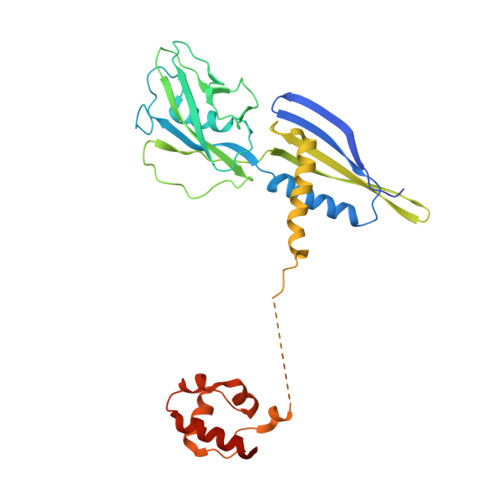
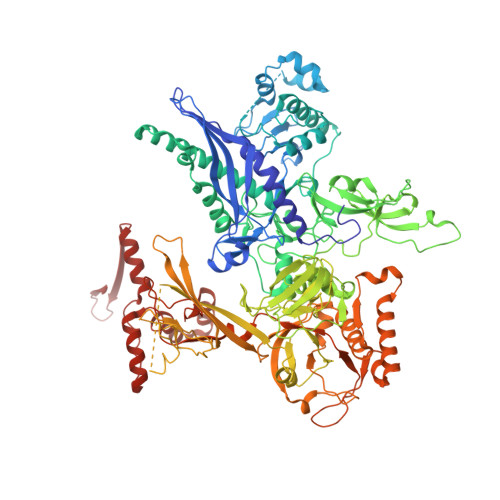
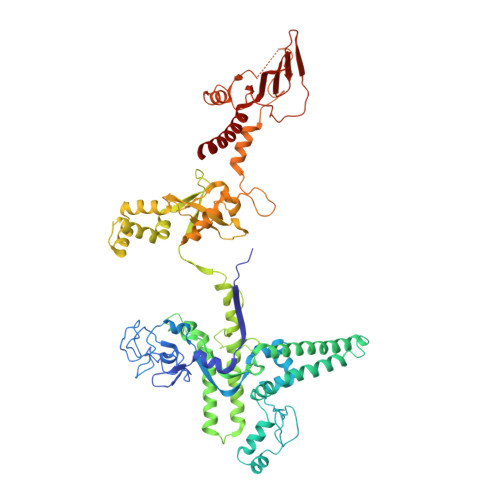
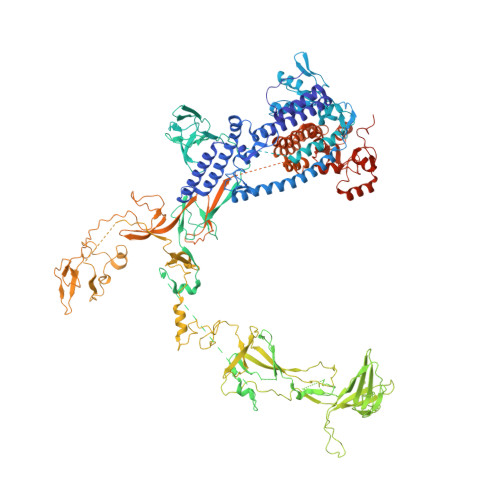
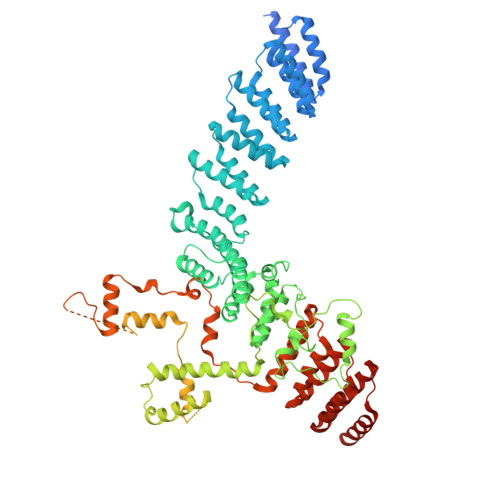
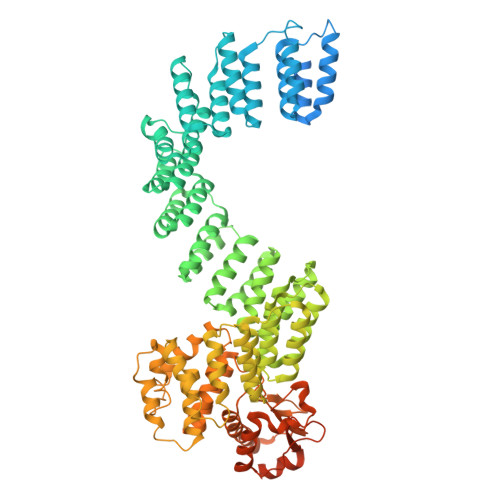
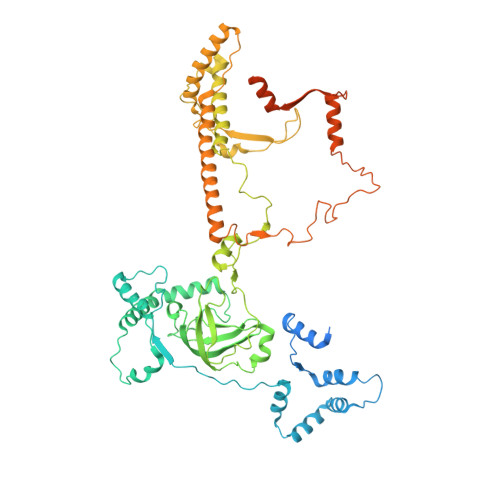
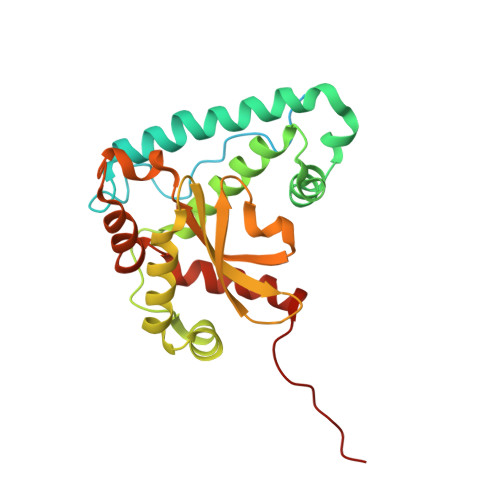
Cryo-EM structures of the plant plastid-encoded RNA polymerase.
Wu, X.X., Mu, W.H., Li, F., Sun, S.Y., Cui, C.J., Kim, C., Zhou, F., Zhang, Y.(2024) Cell 187: 1127-1144.e21
- PubMed: 38428393
- DOI: https://doi.org/10.1016/j.cell.2024.01.026
- Primary Citation of Related Structures:
8W9Z, 8WA0, 8WA1 - PubMed Abstract:
Chloroplasts are green plastids in the cytoplasm of eukaryotic algae and plants responsible for photosynthesis. The plastid-encoded RNA polymerase (PEP) plays an essential role during chloroplast biogenesis from proplastids and functions as the predominant RNA polymerase in mature chloroplasts. The PEP-centered transcription apparatus comprises a bacterial-origin PEP core and more than a dozen eukaryotic-origin PEP-associated proteins (PAPs) encoded in the nucleus. Here, we determined the cryo-EM structures of Nicotiana tabacum (tobacco) PEP-PAP apoenzyme and PEP-PAP transcription elongation complexes at near-atomic resolutions. Our data show the PEP core adopts a typical fold as bacterial RNAP. Fifteen PAPs bind at the periphery of the PEP core, facilitate assembling the PEP-PAP supercomplex, protect the complex from oxidation damage, and likely couple gene transcription with RNA processing. Our results report the high-resolution architecture of the chloroplast transcription apparatus and provide the structural basis for the mechanistic and functional study of transcription regulation in chloroplasts.
Organizational Affiliation:
Key Laboratory of Synthetic Biology, Key Laboratory of Plant Design, CAS Center for Excellence in Molecular Plant Sciences, Shanghai Institute of Plant Physiology and Ecology, Chinese Academy of Sciences, Shanghai 200032, China.