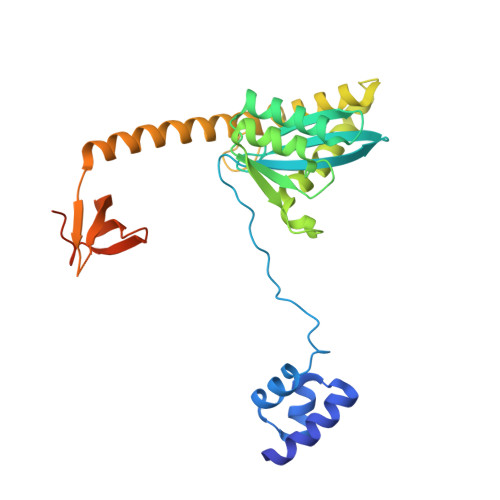
HIV-1 Integrase Assembles Multiple Species of Stable Synaptic Complex Intasomes That Are Active for Concerted DNA Integration In vitro.
Li, M., Yang, R., Chen, X., Wang, H., Ghirlando, R., Dimitriadis, E.K., Craigie, R.(2024) J Mol Biology 436: 168557-168557
- PubMed: 38582148
- DOI: https://doi.org/10.1016/j.jmb.2024.168557
- Primary Citation of Related Structures:
8W09, 8W2R, 8W34 - PubMed Abstract:
Retroviral DNA integration is mediated by nucleoprotein complexes (intasomes) in which a pair of viral DNA ends are bridged by a multimer of integrase (IN). Most of the high-resolution structures of HIV-1 intasomes are based on an HIV-1 IN with an Sso7d protein domain fused to the N-terminus. Sso7d-IN aggregates much less than wild-type IN and has been critical for structural studies of HIV-1 intasomes. Unexpectedly, these structures revealed that the common core architecture that mediates catalysis could be assembled in various ways, giving rise to both tetrameric and dodecameric intasomes, together with other less well-characterized species. This differs from related retroviruses that assemble unique multimeric intasomes, although the number of protomers in the intasome varies between viruses. The question of whether the additional Sso7d domain contributes to the heterogeneity of HIV-1 intasomes is therefore raised. We have addressed this by biochemical and structural studies of intasomes assembled with wild-type HIV-1 IN. Negative stain and cryo-EM reveal a similar range of multimeric intasome species as with Sso7d-IN with the same common core architecture. Stacks of intasomes resulting from domain swapping are also seen with both wild-type and Sso7d-IN intasomes. The propensity to assemble multimeric intasome species is, therefore, an intrinsic property of HIV-1 IN and is not conferred by the presence of the Sso7d domain. The recently solved intasome structures of different retroviral species, which have been reported to be tetrameric, octameric, dodecameric, and hexadecameric, highlight how a common intasome core architecture can be assembled in different ways for catalysis.
- Laboratory of Molecular Biology, NIDDK, National Institutes of Health, Bethesda, MD 20892, USA. Electronic address: lmin@niddk.nih.gov.
Organizational Affiliation: