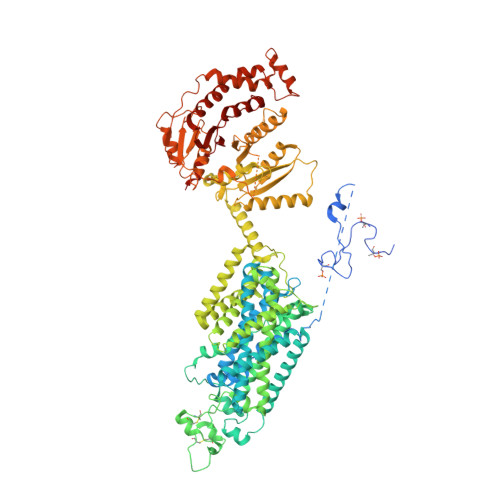
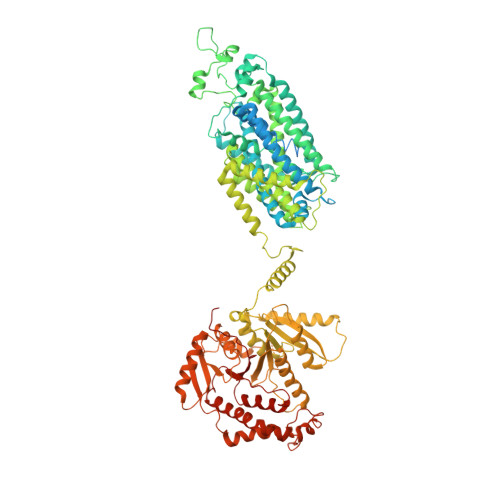
Structural bases for Na + -Cl - cotransporter inhibition by thiazide diuretic drugs and activation by kinases.
Zhao, Y., Schubert, H., Blakely, A., Forbush, B., Smith, M.D., Rinehart, J., Cao, E.(2024) Nat Commun 15: 7006-7006
- PubMed: 39143061
- DOI: https://doi.org/10.1038/s41467-024-51381-y
- Primary Citation of Related Structures:
8VPN, 8VPP, 9BWT - PubMed Abstract:
The Na + -Cl - cotransporter (NCC) drives salt reabsorption in the kidney and plays a decisive role in balancing electrolytes and blood pressure. Thiazide and thiazide-like diuretics inhibit NCC-mediated renal salt retention and have been cornerstones for treating hypertension and edema since the 1950s. Here we determine NCC co-structures individually complexed with the thiazide drug hydrochlorothiazide, and two thiazide-like drugs chlorthalidone and indapamide, revealing that they fit into an orthosteric site and occlude the NCC ion translocation pathway. Aberrant NCC activation by the WNKs-SPAK kinase cascade underlies Familial Hyperkalemic Hypertension, but it remains unknown whether/how phosphorylation transforms the NCC structure to accelerate ion translocation. We show that an intracellular amino-terminal motif of NCC, once phosphorylated, associates with the carboxyl-terminal domain, and together, they interact with the transmembrane domain. These interactions suggest a phosphorylation-dependent allosteric network that directly influences NCC ion translocation.
Organizational Affiliation:
Department of Biochemistry, University of Utah School of Medicine, Salt Lake City, UT, USA.