Structural and functional effects of phosphopriming and scaffolding in the kinase GSK3beta
Enos, M.D., Gavagan, M., Jameson, N., Zalatan, J.G., Weis, W.I.(2024) Sci Signal
Experimental Data Snapshot
Starting Model: experimental
View more details
(2024) Sci Signal
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Glycogen synthase kinase-3 beta | 364 | Mus musculus | Mutation(s): 0 Gene Names: Gsk3b EC: 2.7.11.26 (PDB Primary Data), 2.7.11.1 (PDB Primary Data) | 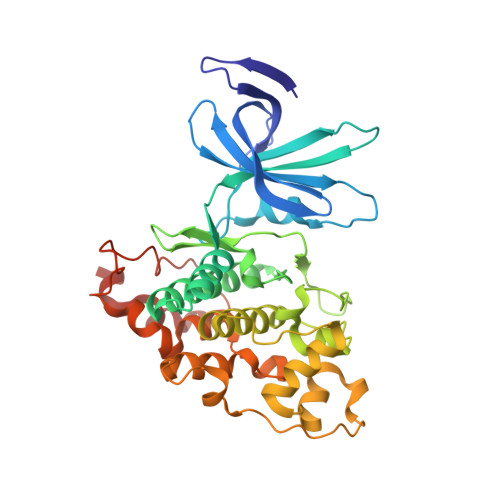 | |
UniProt | |||||
Find proteins for Q9WV60 (Mus musculus) Explore Q9WV60 Go to UniProtKB: Q9WV60 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9WV60 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Axin-1 | 60 | Homo sapiens | Mutation(s): 0 Gene Names: AXIN1, AXIN | 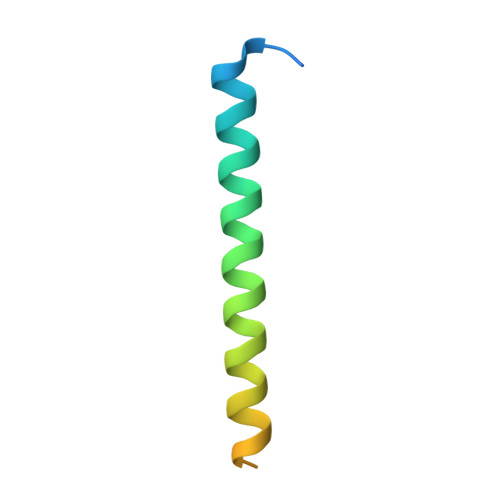 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O15169 (Homo sapiens) Explore O15169 Go to UniProtKB: O15169 | |||||
PHAROS: O15169 GTEx: ENSG00000103126 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O15169 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 8 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ADP Query on ADP | E [auth A] | ADENOSINE-5'-DIPHOSPHATE C10 H15 N5 O10 P2 XTWYTFMLZFPYCI-KQYNXXCUSA-N |  | ||
| MES Query on MES | TA [auth B] | 2-(N-MORPHOLINO)-ETHANESULFONIC ACID C6 H13 N O4 S SXGZJKUKBWWHRA-UHFFFAOYSA-N |  | ||
| SO4 Query on SO4 | AC [auth C] HA [auth A] IA [auth A] JA [auth A] KA [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| GOL Query on GOL | CA [auth A], N [auth A], R [auth A], W [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | AA [auth A] AB [auth B] BA [auth A] BB [auth B] CB [auth B] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| NO3 Query on NO3 | EA [auth A] FA [auth A] GA [auth A] K [auth A] L [auth A] | NITRATE ION N O3 NHNBFGGVMKEFGY-UHFFFAOYSA-N |  | ||
| CL Query on CL | BC [auth C] CC [auth C] DC [auth D] OA [auth A] PA [auth A] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| MG Query on MG | NA [auth A] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 195.585 | α = 90 |
| b = 195.585 | β = 90 |
| c = 152.204 | γ = 120 |
| Software Name | Purpose |
|---|---|
| Blu-Ice | data collection |
| XDS | data reduction |
| pointless | data scaling |
| Aimless | data scaling |
| PHENIX | phasing |
| PHENIX | refinement |
| Coot | model building |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | GM131747 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | GM119156 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | GM007276 |