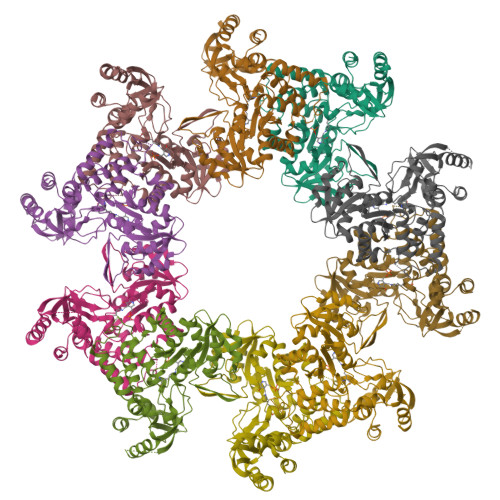
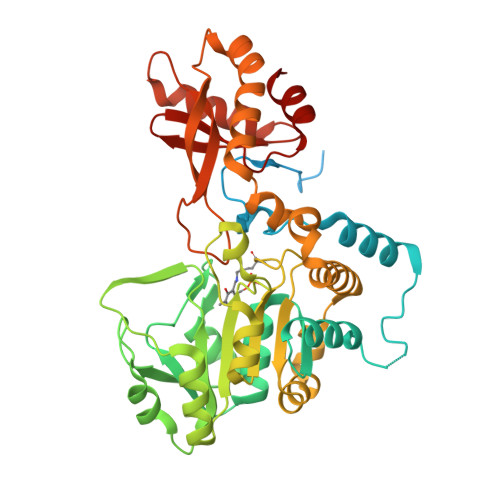
Bacterial selenocysteine synthase structure revealed by single-particle cryoEM.
Balasco Serrao, V.H., Minari, K., Pereira, H.D., Thiemann, O.H.(2024) Curr Res Struct Biol 7: 100143-100143
- PubMed: 38681238
- DOI: https://doi.org/10.1016/j.crstbi.2024.100143
- Primary Citation of Related Structures:
8UZW - PubMed Abstract:
The 21st amino acid, selenocysteine (Sec), is synthesized on its dedicated transfer RNA (tRNA Sec ). In bacteria, Sec is synthesized from Ser-tRNA [Ser]Sec by Selenocysteine Synthase (SelA), which is a pivotal enzyme in the biosynthesis of Sec. The structural characterization of bacterial SelA is of paramount importance to decipher its catalytic mechanism and its role in the regulation of the Sec-synthesis pathway. Here, we present a comprehensive single-particle cryo-electron microscopy (SPA cryoEM) structure of the bacterial SelA with an overall resolution of 2.69 Å. Using recombinant Escherichia coli SelA, we purified and prepared samples for single-particle cryoEM. The structural insights from SelA, combined with previous in vivo and in vitro knowledge, underscore the indispensable role of decamerization in SelA's function. Moreover, our structural analysis corroborates previous results that show that SelA adopts a pentamer of dimers configuration, and the active site architecture, substrate binding pocket, and key K295 catalytic residue are identified and described in detail. The differences in protein architecture and substrate coordination between the bacterial enzyme and its counterparts offer compelling structural evidence supporting the independent molecular evolution of the bacterial and archaea/eukarya Ser-Sec biosynthesis present in the natural world.
Organizational Affiliation:
Biomolecular Cryoelectron Microscopy Facility, University of California - Santa Cruz, Santa Cruz, CA, 95064, United States.