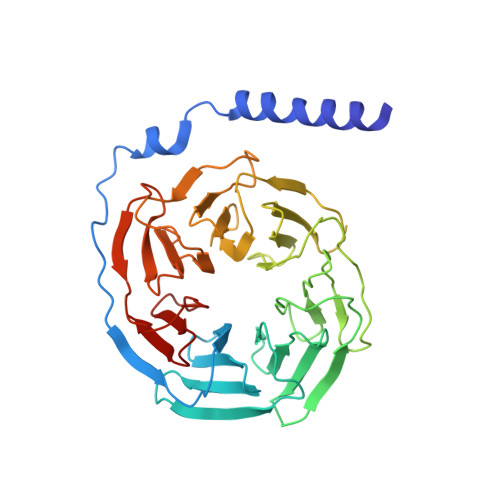
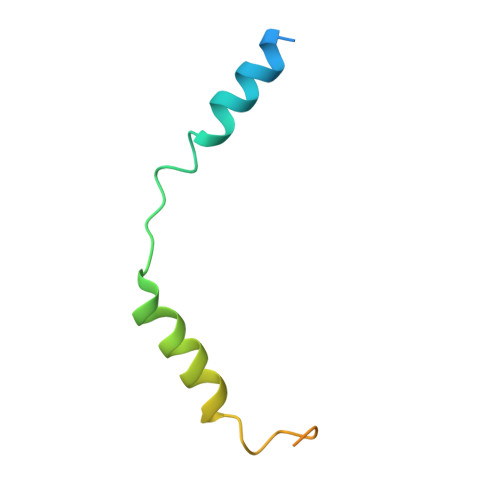
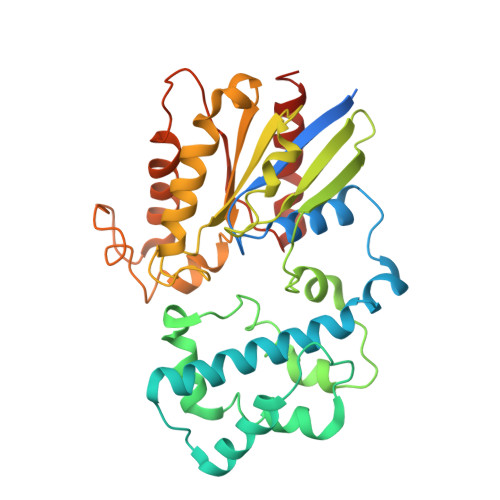
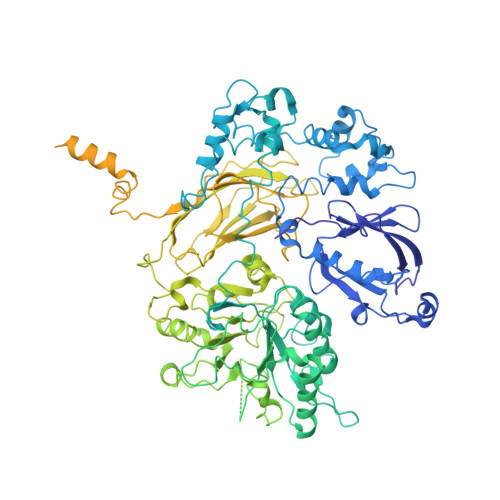
The mechanism of G alpha q regulation of PLC beta 3 -catalyzed PIP2 hydrolysis.
Falzone, M.E., MacKinnon, R.(2023) Proc Natl Acad Sci U S A 120: e2315011120-e2315011120
- PubMed: 37991948
- DOI: https://doi.org/10.1073/pnas.2315011120
- Primary Citation of Related Structures:
8UQN, 8UQO - PubMed Abstract:
PLCβ ( Phospholipase Cβ ) enzymes cleave phosphatidylinositol 4,5-bisphosphate ( PIP2) producing IP3 and DAG (diacylglycerol). PIP2 modulates the function of many ion channels, while IP3 and DAG regulate intracellular Ca 2+ levels and protein phosphorylation by protein kinase C, respectively. PLCβ enzymes are under the control of G protein coupled receptor signaling through direct interactions with G proteins Gβγ and Gα q and have been shown to be coincidence detectors for dual stimulation of Gα q and Gα i -coupled receptors. PLCβs are aqueous-soluble cytoplasmic enzymes but partition onto the membrane surface to access their lipid substrate, complicating their functional and structural characterization. Using newly developed methods, we recently showed that Gβγ activates PLCβ3 by recruiting it to the membrane. Using these same methods, here we show that Gα q increases the catalytic rate constant, k cat , of PLCβ3 . Since stimulation of PLCβ3 by Gα q depends on an autoinhibitory element (the X-Y linker), we propose that Gα q produces partial relief of the X-Y linker autoinhibition through an allosteric mechanism. We also determined membrane-bound structures of the PLCβ3·Gα q and PLCβ3·Gβγ (2) ·Gα q complexes, which show that these G proteins can bind simultaneously and independently of each other to regulate PLCβ3 activity. The structures rationalize a finding in the enzyme assay, that costimulation by both G proteins follows a product rule of each independent stimulus. We conclude that baseline activity of PLCβ3 is strongly suppressed, but the effect of G proteins, especially acting together, provides a robust stimulus upon G protein stimulation.
Organizational Affiliation:
Laboratory of Molecular Neurobiology and Biophysics, The Rockefeller University, New York, NY 10065.