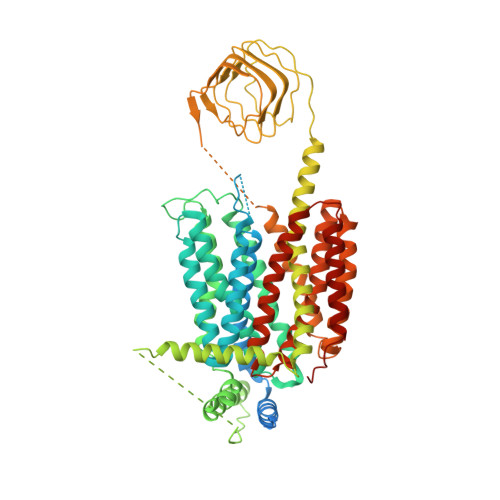
Structures of synaptic vesicle protein 2A and 2B bound to anticonvulsants.
Mittal, A., Martin, M.F., Levin, E.J., Adams, C., Yang, M., Provins, L., Hall, A., Procter, M., Ledecq, M., Hillisch, A., Wolff, C., Gillard, M., Horanyi, P.S., Coleman, J.A.(2024) Nat Struct Mol Biol
- PubMed: 38898101
- DOI: https://doi.org/10.1038/s41594-024-01335-1
- Primary Citation of Related Structures:
8UO8, 8UO9, 8UOA - PubMed Abstract:
Epilepsy is a common neurological disorder characterized by abnormal activity of neuronal networks, leading to seizures. The racetam class of anti-seizure medications bind specifically to a membrane protein found in the synaptic vesicles of neurons called synaptic vesicle protein 2 (SV2) A (SV2A). SV2A belongs to an orphan subfamily of the solute carrier 22 organic ion transporter family that also includes SV2B and SV2C. The molecular basis for how anti-seizure medications act on SV2s remains unknown. Here we report cryo-electron microscopy structures of SV2A and SV2B captured in a luminal-occluded conformation complexed with anticonvulsant ligands. The conformation bound by anticonvulsants resembles an inhibited transporter with closed luminal and intracellular gates. Anticonvulsants bind to a highly conserved central site in SV2s. These structures provide blueprints for future drug design and will facilitate future investigations into the biological function of SV2s.
Organizational Affiliation:
Department of Structural Biology, University of Pittsburgh, Pittsburgh, PA, USA.