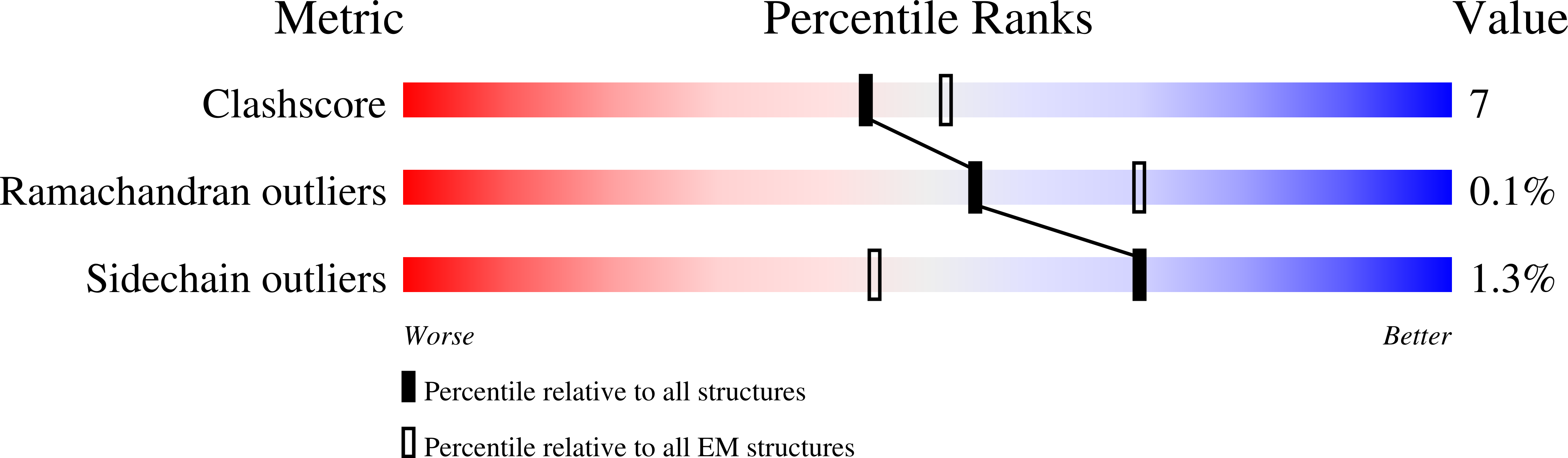Pharmacology of LRRK2 with type I and II kinase inhibitors revealed by cryo-EM.
Zhu, H., Hixson, P., Ma, W., Sun, J.(2024) Cell Discov 10: 10-10
- PubMed: 38263358
- DOI: https://doi.org/10.1038/s41421-023-00639-8
- Primary Citation of Related Structures:
8FO7, 8U7H, 8U7L, 8U8A, 8U8B - PubMed Abstract:
LRRK2 is one of the most promising drug targets for Parkinson's disease. Though type I kinase inhibitors of LRRK2 are under clinical trials, alternative strategies like type II inhibitors are being actively pursued due to the potential undesired effects of type I inhibitors. Currently, a robust method for LRRK2-inhibitor structure determination to guide structure-based drug discovery is lacking, and inhibition mechanisms of available compounds are also unclear. Here we present near-atomic-resolution structures of LRRK2 with type I (LRRK2-IN-1 and GNE-7915) and type II (rebastinib, ponatinib, and GZD-824) inhibitors, uncovering the structural basis of LRRK2 inhibition and conformational plasticity of the kinase domain with molecular dynamics (MD) simulations. Type I and II inhibitors bind to LRRK2 in active-like and inactive conformations, so LRRK2-inhibitor complexes further reveal general structural features associated with LRRK2 activation. Our study provides atomic details of LRRK2-inhibitor interactions and a framework for understanding LRRK2 activation and for rational drug design.
Organizational Affiliation:
Department of Structural Biology, St. Jude Children's Research Hospital, Memphis, TN, USA.