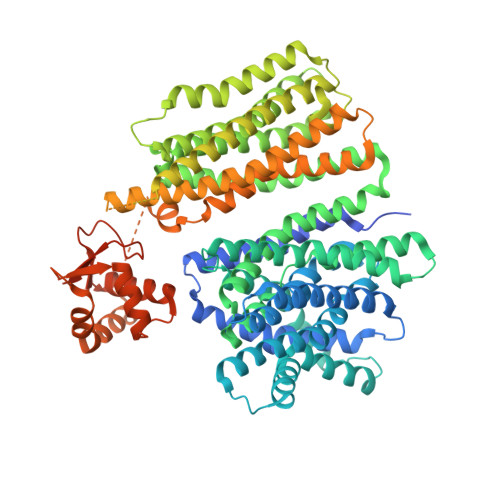
LYCHOS is a human hybrid of a plant-like PIN transporter and a GPCR.
Bayly-Jones, C., Lupton, C.J., Keen, A.C., Dong, S., Mastos, C., Luo, W., Qian, C., Jones, G.D., Venugopal, H., Chang, Y.G., Clarke, R.J., Halls, M.L., Ellisdon, A.M.(2024) Nature 634: 1238-1244
- PubMed: 39358511
- DOI: https://doi.org/10.1038/s41586-024-08012-9
- Primary Citation of Related Structures:
8U54, 8U56, 8U58, 8U5C, 8U5N, 8U5Q, 8U5V, 8U5X - PubMed Abstract:
Lysosomes have crucial roles in regulating eukaryotic metabolism and cell growth by acting as signalling platforms to sense and respond to changes in nutrient and energy availability 1 . LYCHOS (GPR155) is a lysosomal transmembrane protein that functions as a cholesterol sensor, facilitating the cholesterol-dependent activation of the master protein kinase mechanistic target of rapamycin complex 1 (mTORC1) 2 . However, the structural basis of LYCHOS assembly and activity remains unclear. Here we determine several high-resolution cryo-electron microscopy structures of human LYCHOS, revealing a homodimeric transmembrane assembly of a transporter-like domain fused to a G-protein-coupled receptor (GPCR) domain. The class B2-like GPCR domain is captured in the apo state and packs against the surface of the transporter-like domain, providing an unusual example of a GPCR as a domain in a larger transmembrane assembly. Cholesterol sensing is mediated by a conserved cholesterol-binding motif, positioned between the GPCR and transporter domains. We reveal that the LYCHOS transporter-like domain is an orthologue of the plant PIN-FORMED (PIN) auxin transporter family, and has greater structural similarity to plant auxin transporters than to known human transporters. Activity assays support a model in which the LYCHOS transporter and GPCR domains coordinate to sense cholesterol and regulate mTORC1 activation.
Organizational Affiliation:
Cancer Program, Biomedicine Discovery Institute, Monash University, Clayton, Victoria, Australia.