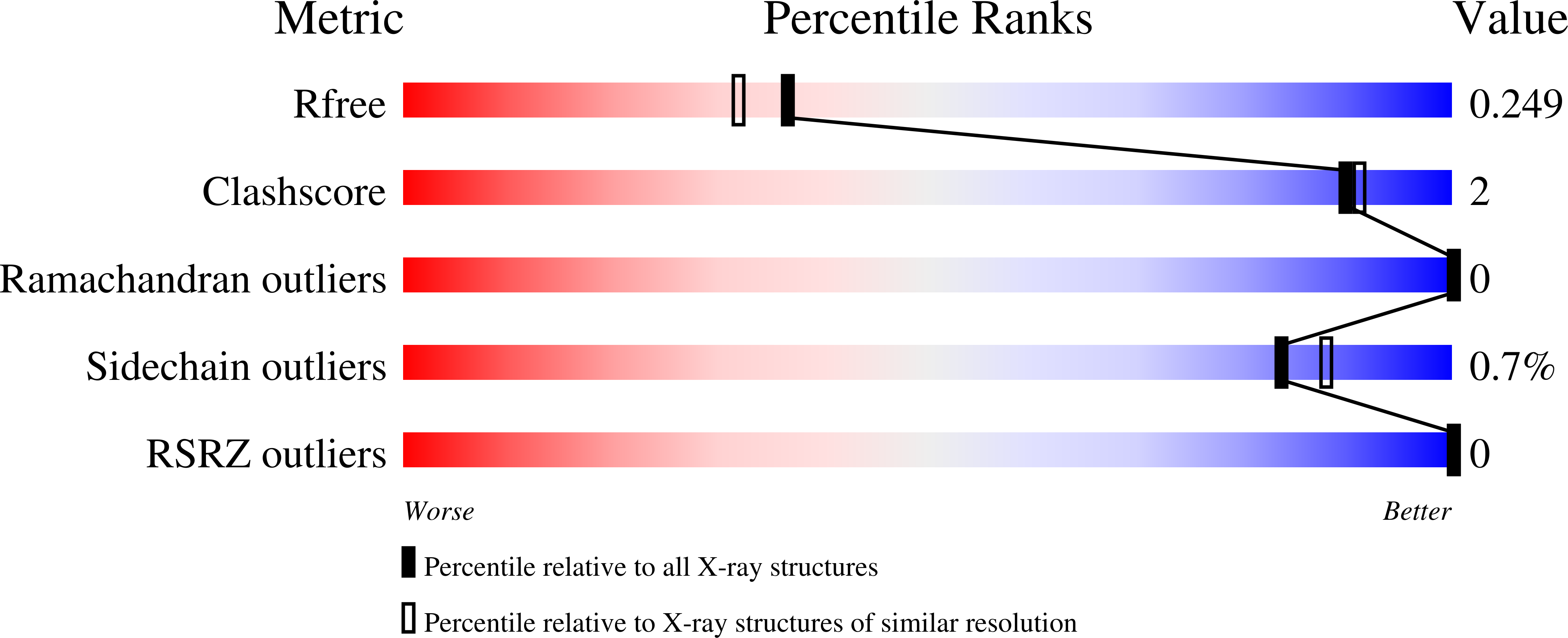
Phages overcome bacterial immunity via diverse anti-defence proteins.
Yirmiya, E., Leavitt, A., Lu, A., Ragucci, A.E., Avraham, C., Osterman, I., Garb, J., Antine, S.P., Mooney, S.E., Hobbs, S.J., Kranzusch, P.J., Amitai, G., Sorek, R.(2024) Nature 625: 352-359
- PubMed: 37992756
- DOI: https://doi.org/10.1038/s41586-023-06869-w
- Primary Citation of Related Structures:
8SMD, 8SME, 8SMF, 8SMG, 8TTO - PubMed Abstract:
It was recently shown that bacteria use, apart from CRISPR-Cas and restriction systems, a considerable diversity of phage resistance systems 1-4 , but it is largely unknown how phages cope with this multilayered bacterial immunity. Here we analysed groups of closely related Bacillus phages that showed differential sensitivity to bacterial defence systems, and discovered four distinct families of anti-defence proteins that inhibit the Gabija, Thoeris and Hachiman systems. We show that these proteins Gad1, Gad2, Tad2 and Had1 efficiently cancel the defensive activity when co-expressed with the respective defence system or introduced into phage genomes. Homologues of these anti-defence proteins are found in hundreds of phages that infect taxonomically diverse bacterial species. We show that the anti-Gabija protein Gad1 blocks the ability of the Gabija defence complex to cleave phage-derived DNA. Our data further reveal that the anti-Thoeris protein Tad2 is a 'sponge' that sequesters the immune signalling molecules produced by Thoeris TIR-domain proteins in response to phage infection. Our results demonstrate that phages encode an arsenal of anti-defence proteins that can disable a variety of bacterial defence mechanisms.
Organizational Affiliation:
Department of Molecular Genetics, Weizmann Institute of Science, Rehovot, Israel.