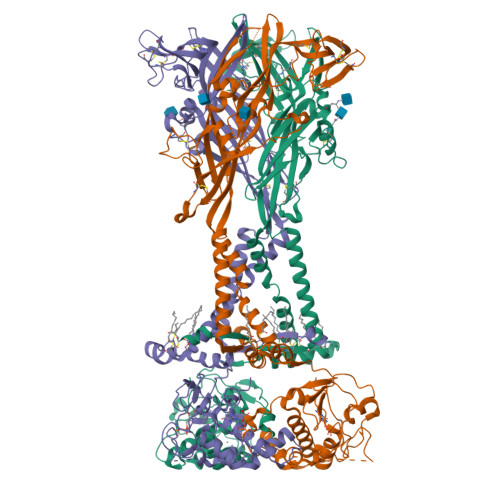
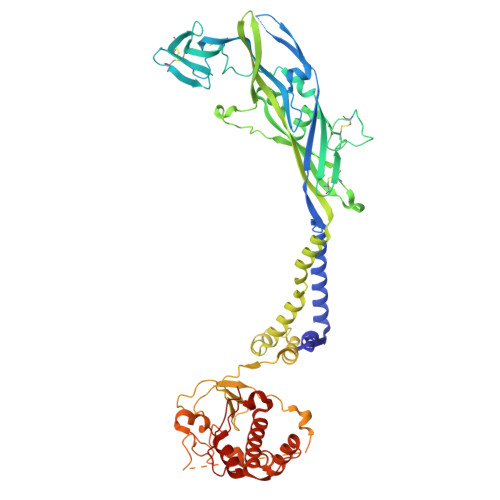
P2X 7 receptors exhibit at least three modes of allosteric antagonism.
Oken, A.C., Ditter, I.A., Lisi, N.E., Krishnamurthy, I., Godsey, M.H., Mansoor, S.E.(2024) Sci Adv 10: eado5084-eado5084
- PubMed: 39365862
- DOI: https://doi.org/10.1126/sciadv.ado5084
- Primary Citation of Related Structures:
8TR6, 8TR7, 8TR8, 8TRA, 8TRB, 8TRK - PubMed Abstract:
P2X receptors are trimeric ion channels activated by adenosine triphosphate (ATP) that contribute to pathophysiological processes ranging from asthma to neuropathic pain and neurodegeneration. A number of small-molecule antagonists have been identified for these important pharmaceutical targets. However, the molecular pharmacology of P2X receptors is poorly understood because of the chemically disparate nature of antagonists and their differential actions on the seven constituent subtypes. Here, we report high-resolution cryo-electron microscopy structures of the homomeric rat P2X 7 receptor bound to five previously known small-molecule allosteric antagonists and a sixth antagonist that we identify. Our structural, biophysical, and electrophysiological data define the molecular determinants of allosteric antagonism in this pharmacologically relevant receptor, revealing three distinct classes of antagonists that we call shallow, deep, and starfish. Starfish binders, exemplified by the previously unidentified antagonist methyl blue, represent a unique class of inhibitors with distinct functional properties that could be exploited to develop potent P2X 7 ligands with substantial clinical impact.
Organizational Affiliation:
Department of Chemical Physiology and Biochemistry, Oregon Health & Science University, Portland, OR 97239, USA.