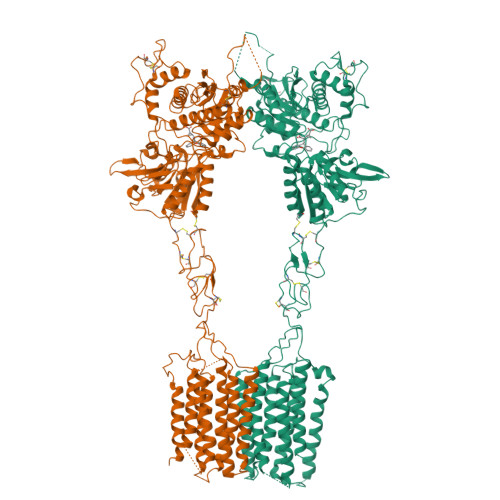
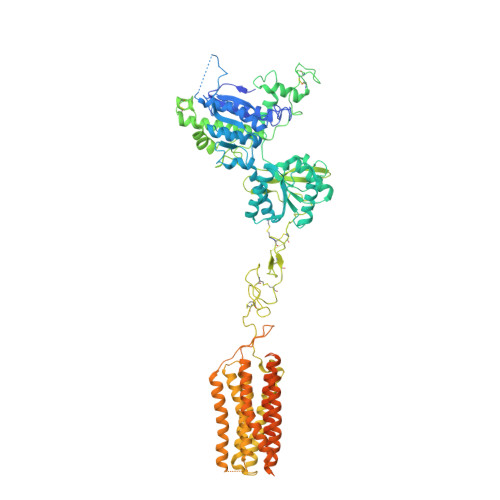
Structural basis of allosteric modulation of metabotropic glutamate receptor activation and desensitization.
Strauss, A., Gonzalez-Hernandez, A.J., Lee, J., Abreu, N., Selvakumar, P., Salas-Estrada, L., Kristt, M., Marx, D.C., Gilliland, K., Melancon, B.J., Filizola, M., Meyerson, J., Levitz, J.(2023) bioRxiv
- PubMed: 37645747
- DOI: https://doi.org/10.1101/2023.08.13.552748
- Primary Citation of Related Structures:
8TQB, 8TR0, 8TR2, 8TRC - PubMed Abstract:
The metabotropic glutamate receptors (mGluRs) are neuromodulatory family C G protein coupled receptors which assemble as dimers and allosterically couple extracellular ligand binding domains (LBDs) to transmembrane domains (TMDs) to drive intracellular signaling. Pharmacologically, mGluRs can be targeted either at the LBDs by glutamate and synthetic orthosteric compounds or at the TMDs by allosteric modulators. Despite the potential of allosteric TMD-targeting compounds as therapeutics, an understanding of the functional and structural basis of their effects on mGluRs is limited. Here we use a battery of approaches to dissect the distinct functional and structural effects of orthosteric versus allosteric ligands. We find using electrophysiological and live cell imaging assays that both agonists and positive allosteric modulators (PAMs) can drive activation and desensitization of mGluRs. The effects of PAMs are pleiotropic, including both the ability to boost the maximal response to orthosteric agonists and to serve independently as desensitization-biased agonists across mGluR subtypes. Conformational sensors reveal PAM-driven inter-subunit re-arrangements at both the LBD and TMD. Motivated by this, we determine cryo-electron microscopy structures of mGluR3 in the presence of either an agonist or antagonist alone or in combination with a PAM. These structures reveal PAM-driven re-shaping of intra- and inter-subunit conformations and provide evidence for a rolling TMD dimer interface activation pathway that controls G protein and beta-arrestin coupling. -Agonists and PAMs drive mGluR activation, desensitization, and endocytosis-PAMs are desensitization-biased and synergistic with agonists-Four combinatorial ligand conditions reveal an ensemble of full-length mGluR structures with novel interfaces-Activation and desensitization involve rolling TMD interfaces which are re-shaped by PAM.