Molecular basis of the mode of interaction between the tandem LC3-interacting region motif of the autophagy tethering factor EPG5 and ATG8 proteins
Cheung, Y.W.S., Fairlie, G., Scheu, K., Yip, C.K.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protein lgg-1 | 125 | Caenorhabditis elegans | Mutation(s): 0 Gene Names: lgg-1, atg-8.1, C32D5.9 | 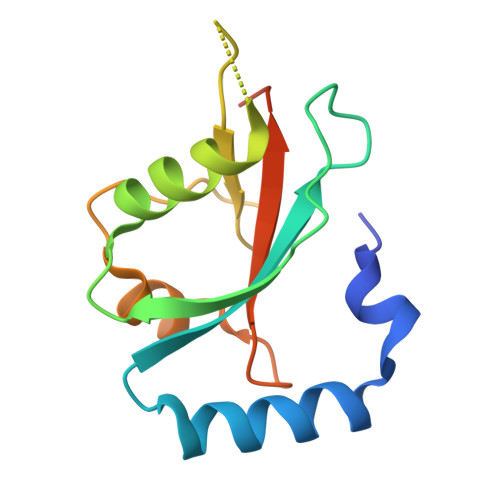 | |
UniProt | |||||
Find proteins for Q09490 (Caenorhabditis elegans) Explore Q09490 Go to UniProtKB: Q09490 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q09490 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ectopic P granules protein 5 | 11 | Caenorhabditis elegans | Mutation(s): 0 | 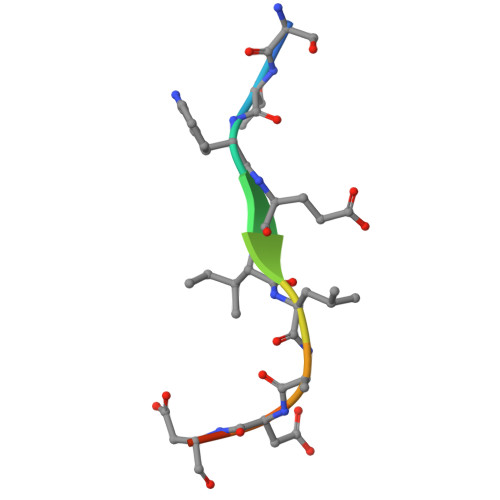 | |
UniProt | |||||
Find proteins for Q18892 (Caenorhabditis elegans) Explore Q18892 Go to UniProtKB: Q18892 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q18892 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ACT Query on ACT | C [auth A] | ACETATE ION C2 H3 O2 QTBSBXVTEAMEQO-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 33.991 | α = 90 |
| b = 37.952 | β = 100.102 |
| c = 45.177 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DIALS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| PHENIX | refinement |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Canadian Institutes of Health Research (CIHR) | Canada | -- |