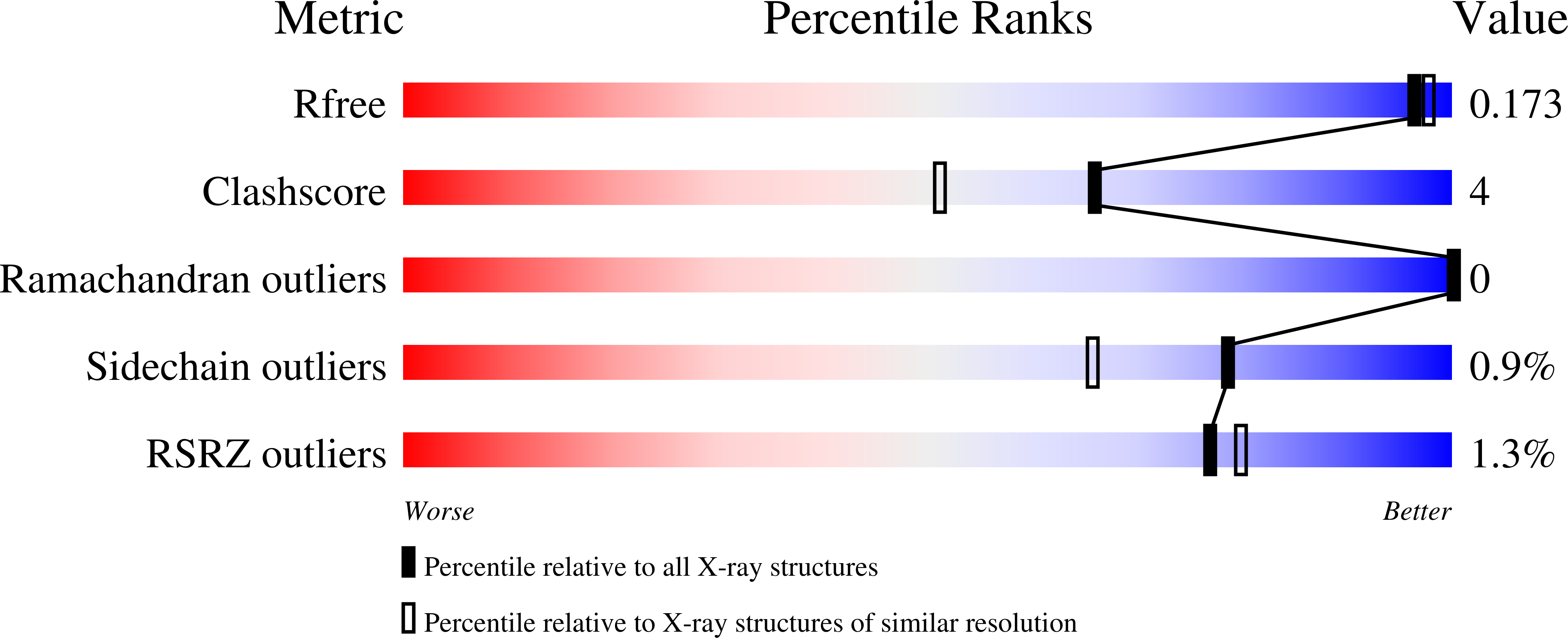
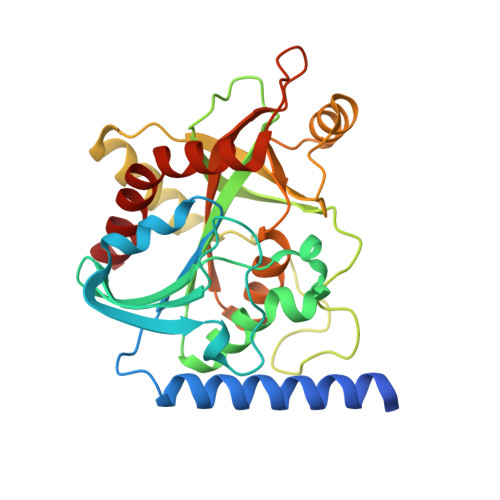
Phosphate Binding in PNP Alters Transition-State Analogue Affinity and Subunit Cooperativity.
Minnow, Y.V.T., Schramm, V.L., Almo, S.C., Ghosh, A.(2023) Biochemistry 62: 3116-3125
- PubMed: 37812583
- DOI: https://doi.org/10.1021/acs.biochem.3c00264
- Primary Citation of Related Structures:
8SWP, 8SWQ, 8SWR, 8SWS, 8SWT, 8SWU - PubMed Abstract:
Purine nucleoside phosphorylases (PNPs) catalyze the phosphorolysis of 6-oxypurine nucleosides with an HPO 4 2- dianion nucleophile. Nucleosides and phosphate occupy distinct pockets in the PNP active site. Evaluation of the HPO 4 2- site by mutagenesis, cooperative binding studies, and thermodynamic and structural analysis demonstrate that alterations in the HPO 4 2- binding site can render PNP inactive and significantly impact subunit cooperativity and binding to transition-state analogue inhibitors. Cooperative interactions between the cationic transition-state analogue and the anionic HPO 4 2- nucleophile demonstrate the importance of reforming the transition-state ensemble for optimal inhibition with transition-state analogues. Altered phosphate binding in the catalytic site mutants helps to explain one of the known lethal PNP deficiency syndromes in humans.
Organizational Affiliation:
Department of Biochemistry, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, New York 10461, United States.