Crystal structure of TuUGT202A2 (Tetur22g00270) in complex with naringin
Arriaza, R.H., Dermauw, W., Wybouw, N., Van Leeuwen, T., Chruszcz, M.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| UDP-glycosyltransferase 202A2 | 437 | Tetranychus urticae | Mutation(s): 0 Gene Names: 107367435, UGT202A2 | 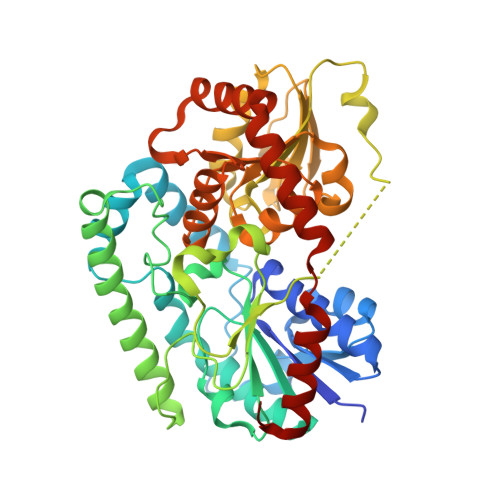 | |
UniProt | |||||
Find proteins for T1KUK4 (Tetranychus urticae) Explore T1KUK4 Go to UniProtKB: T1KUK4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | T1KUK4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ZWN (Subject of Investigation/LOI) Query on ZWN | D [auth A], F [auth B] | (2S)-5-hydroxy-2-(4-hydroxyphenyl)-4-oxo-3,4-dihydro-2H-1-benzopyran-7-yl 2-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranoside C27 H32 O14 DFPMSGMNTNDNHN-ZPHOTFPESA-N |  | ||
| UDP (Subject of Investigation/LOI) Query on UDP | C [auth A], E [auth B] | URIDINE-5'-DIPHOSPHATE C9 H14 N2 O12 P2 XCCTYIAWTASOJW-XVFCMESISA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 47.482 | α = 90 |
| b = 115.677 | β = 90 |
| c = 165.693 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |
| MOLREP | phasing |
| SERGUI | data collection |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institute of Food and Agriculture (NIFA, United States) | United States | #2020-67014- 31179 |