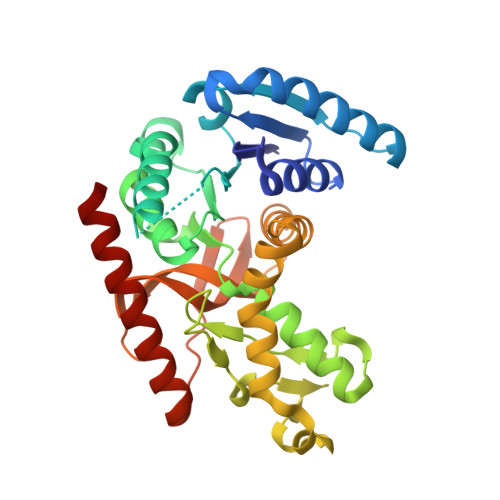
Navigating the conformational landscape of an enzyme. Stabilization of a low populated conformer by evolutionary mutations triggers Allostery into a non-allosteric enzyme.
Coquille, S., Simoes Pereira, C., Brochier-Armanet, C., Roche, J., Santoni, G., Coquelle, N., Girard, E., Sterpone, F., Madern, D.To be published.