The Cohesin ATPase cycle is mediated by specific conformational dynamics and interface plasticity of SMC1A and SMC3 ATPase domains
Vitoria Gomes, M., Romier, C.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Structural maintenance of chromosomes protein 3 | 462 | Homo sapiens | Mutation(s): 0 Gene Names: SMC3, BAM, BMH, CSPG6, SMC3L1 | 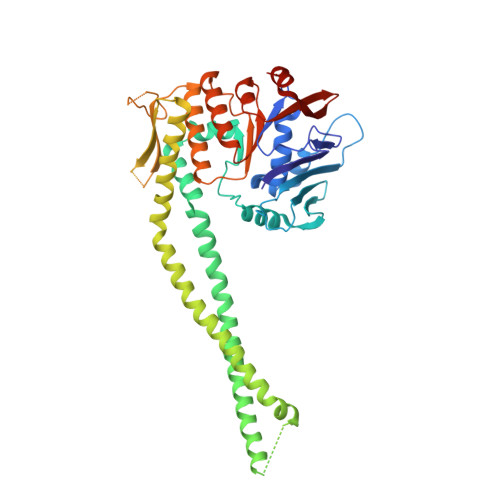 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9UQE7 (Homo sapiens) Explore Q9UQE7 Go to UniProtKB: Q9UQE7 | |||||
PHAROS: Q9UQE7 GTEx: ENSG00000108055 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9UQE7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Double-strand-break repair protein rad21 homolog | 110 | Homo sapiens | Mutation(s): 0 Gene Names: RAD21, HR21, KIAA0078, NXP1, SCC1 | 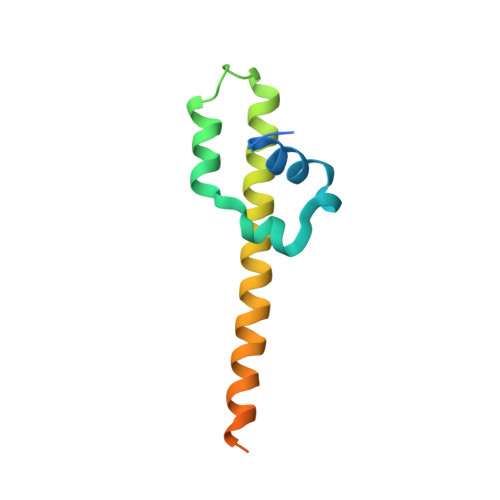 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O60216 (Homo sapiens) Explore O60216 Go to UniProtKB: O60216 | |||||
PHAROS: O60216 GTEx: ENSG00000164754 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O60216 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ADP (Subject of Investigation/LOI) Query on ADP | D [auth A] | ADENOSINE-5'-DIPHOSPHATE C10 H15 N5 O10 P2 XTWYTFMLZFPYCI-KQYNXXCUSA-N |  | ||
| MG (Subject of Investigation/LOI) Query on MG | C [auth A] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 89.706 | α = 90 |
| b = 89.706 | β = 90 |
| c = 234.645 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| XDS | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Fondation ARC | France | ARCPJA20181208268 |
| Fondation ARC | France | ARCPJA2021060003715 |
| Fondation ARC | France | DOC20180507150 |
| Agence Nationale de la Recherche (ANR) | France | ANR-10-LABX-030-INRT |
| Agence Nationale de la Recherche (ANR) | France | ANR-10-IDEX-0002 |
| Agence Nationale de la Recherche (ANR) | France | ANR-17-EURE-0023 |
| Agence Nationale de la Recherche (ANR) | France | ANR-10-INBS-0005-01 |