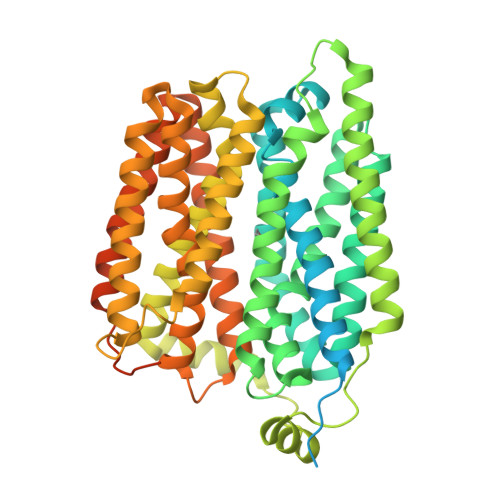
Molecular mechanism of choline and ethanolamine transport in humans.
Ri, K., Weng, T.H., Claveras Cabezudo, A., Josting, W., Zhang, Y., Bazzone, A., Leong, N.C.P., Welsch, S., Doty, R.T., Gursu, G., Lim, T.J.Y., Schmidt, S.L., Abkowitz, J.L., Hummer, G., Wu, D., Nguyen, L.N., Safarian, S.(2024) Nature 630: 501-508
- PubMed: 38778100
- DOI: https://doi.org/10.1038/s41586-024-07444-7
- Primary Citation of Related Structures:
8QCS, 8QCT, 8QCX, 8QCY, 8QD0, 8R8T - PubMed Abstract:
Human feline leukaemia virus subgroup C receptor-related proteins 1 and 2 (FLVCR1 and FLVCR2) are members of the major facilitator superfamily 1 . Their dysfunction is linked to several clinical disorders, including PCARP, HSAN and Fowler syndrome 2-7 . Earlier studies concluded that FLVCR1 may function as a haem exporter 8-12 , whereas FLVCR2 was suggested to act as a haem importer 13 , yet conclusive biochemical and detailed molecular evidence remained elusive for the function of both transporters 14-16 . Here, we show that FLVCR1 and FLVCR2 facilitate the transport of choline and ethanolamine across the plasma membrane, using a concentration-driven substrate translocation process. Through structural and computational analyses, we have identified distinct conformational states of FLVCRs and unravelled the coordination chemistry underlying their substrate interactions. Fully conserved tryptophan and tyrosine residues form the binding pocket of both transporters and confer selectivity for choline and ethanolamine through cation-π interactions. Our findings clarify the mechanisms of choline and ethanolamine transport by FLVCR1 and FLVCR2, enhance our comprehension of disease-associated mutations that interfere with these vital processes and shed light on the conformational dynamics of these major facilitator superfamily proteins during the transport cycle.
Organizational Affiliation:
Department of Biochemistry, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.