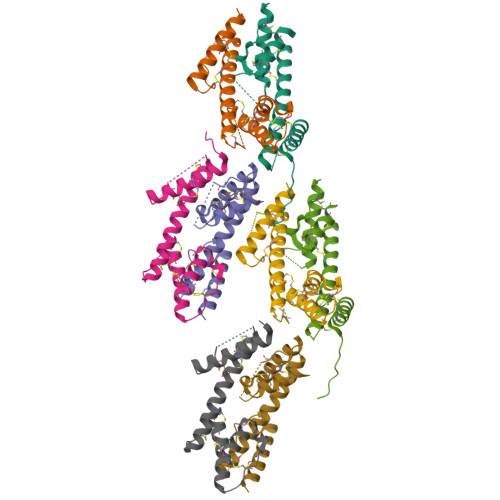
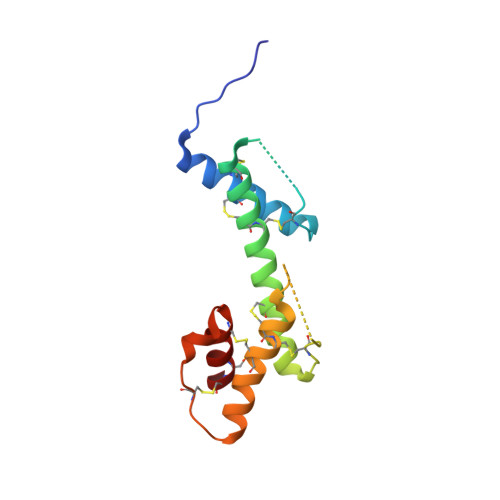
Biophysical and structural studies of fibulin-2.
Sohail, A.A., Koski, M.K., Ruddock, L.W.(2024) Sci Rep 14: 15091-15091
- PubMed: 38956220
- DOI: https://doi.org/10.1038/s41598-024-64931-7
- Primary Citation of Related Structures:
8R5W - PubMed Abstract:
Fibulin-2 is a multidomain, disulfide-rich, homodimeric protein which belongs to a broader extracellular matrix family. It plays an important role in the development of elastic fiber structures. Malfunction of fibulin due to mutation or poor expression can result in a variety of diseases including synpolydactyly, limb abnormalities, eye disorders leading to blindness, cardiovascular diseases and cancer. Traditionally, fibulins have either been produced in mammalian cell systems or were isolated from the extracellular matrix, a procedure that results in poor availability for structural and functional studies. Here, we produced seven fibulin-2 constructs covering 62% of the mature protein (749 out of 1195 residues) using a prokaryotic expression system. Biophysical studies confirm that the purified constructs are folded and that the presence of disulfide bonds within the constructs makes them extremely thermostable. In addition, we solved the first crystal structure for any fibulin isoform, a structure corresponding to the previously suggested three motifs related to anaphylatoxin. The structure reveals that the three anaphylatoxins moieties form a single-domain structure.
Organizational Affiliation:
Faculty of Biochemistry and Molecular Medicine, University of Oulu, 90220, Oulu, Finland.