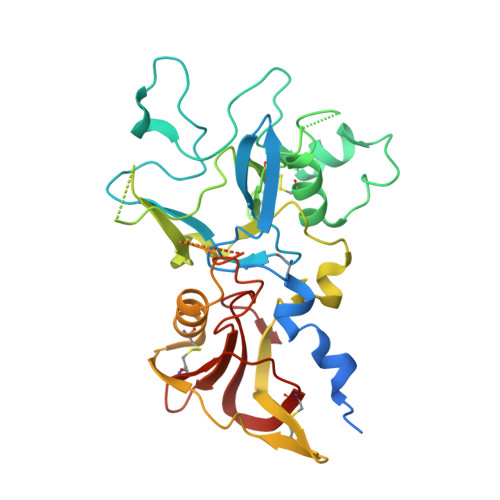
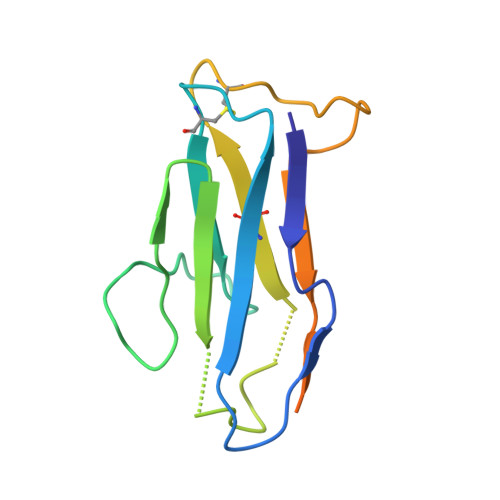
A broadly cross-reactive i-body to AMA1 potently inhibits blood and liver stages of Plasmodium parasites.
Angage, D., Chmielewski, J., Maddumage, J.C., Hesping, E., Caiazzo, S., Lai, K.H., Yeoh, L.M., Menassa, J., Opi, D.H., Cairns, C., Puthalakath, H., Beeson, J.G., Kvansakul, M., Boddey, J.A., Wilson, D.W., Anders, R.F., Foley, M.(2024) Nat Commun 15: 7206-7206
- PubMed: 39174515
- DOI: https://doi.org/10.1038/s41467-024-50770-7
- Primary Citation of Related Structures:
8QU7, 8QUS - PubMed Abstract:
Apical membrane antigen-1 (AMA1) is a conserved malarial vaccine candidate essential for the formation of tight junctions with the rhoptry neck protein (RON) complex, enabling Plasmodium parasites to invade human erythrocytes, hepatocytes, and mosquito salivary glands. Despite its critical role, extensive surface polymorphisms in AMA1 have led to strain-specific protection, limiting the success of AMA1-based interventions beyond initial clinical trials. Here, we identify an i-body, a humanised single-domain antibody-like molecule that recognises a conserved pan-species conformational epitope in AMA1 with low nanomolar affinity and inhibits the binding of the RON2 ligand to AMA1. Structural characterisation indicates that the WD34 i-body epitope spans the centre of the conserved hydrophobic cleft in AMA1, where interacting residues are highly conserved among all Plasmodium species. Furthermore, we show that WD34 inhibits merozoite invasion of erythrocytes by multiple Plasmodium species and hepatocyte invasion by P. falciparum sporozoites. Despite a short half-life in mouse serum, we demonstrate that WD34 transiently suppressed P. berghei infections in female BALB/c mice. Our work describes the first pan-species AMA1 biologic with inhibitory activity against multiple life-cycle stages of Plasmodium. With improved pharmacokinetic characteristics, WD34 could be a potential immunotherapy against multiple species of Plasmodium.
Organizational Affiliation:
Department of Biochemistry and Chemistry, La Trobe Institute for Molecular Sciences, La Trobe University, Victoria, 3086, Australia.