Cryo-EM structure of human SLC15A4 dimer in outward open state in LMNG
Rehan, S., Matsuoka, R., Jazayeri, A., Duerr, K.L.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Solute carrier family 15 member 4 | A, B [auth C] | 577 | Homo sapiens | Mutation(s): 2 Gene Names: SLC15A4 Membrane Entity: Yes | 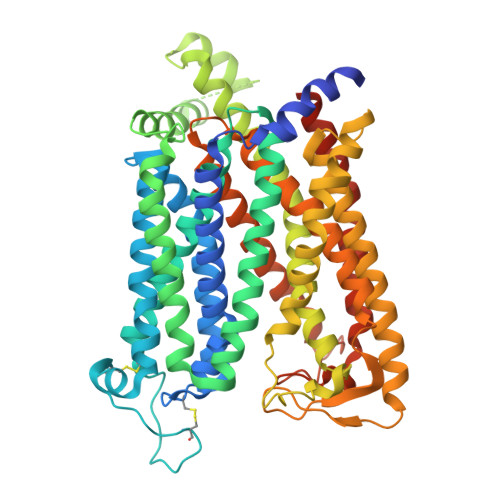 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q8N697 (Homo sapiens) Explore Q8N697 Go to UniProtKB: Q8N697 | |||||
PHAROS: Q8N697 GTEx: ENSG00000139370 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8N697 | ||||
Glycosylation | |||||
| Glycosylation Sites: 1 | Go to GlyGen: Q8N697-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PGT Query on PGT | J [auth A], R [auth C] | (1S)-2-{[{[(2R)-2,3-DIHYDROXYPROPYL]OXY}(HYDROXY)PHOSPHORYL]OXY}-1-[(PALMITOYLOXY)METHYL]ETHYL STEARATE C40 H79 O10 P KBPVYRBBONZJHF-AMAPPZPBSA-N |  | ||
| Y01 Query on Y01 | C [auth A] D [auth A] E [auth A] G [auth A] H [auth A] | CHOLESTEROL HEMISUCCINATE C31 H50 O4 WLNARFZDISHUGS-MIXBDBMTSA-N |  | ||
| NAG Query on NAG | F [auth A], N [auth C] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | cryoSPARC |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Other private | -- |