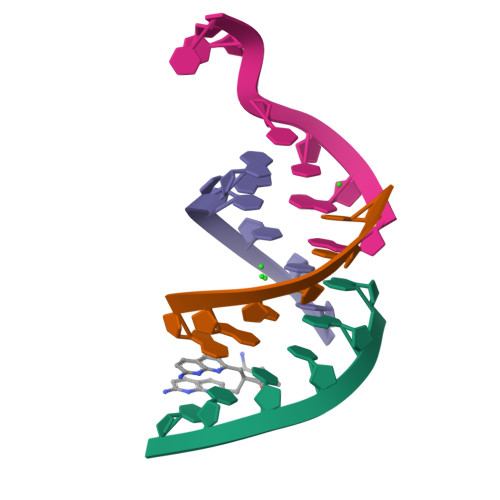
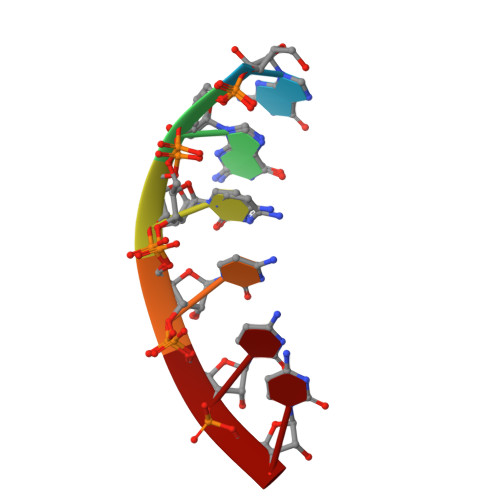
Antisense RNA C9orf72 hexanucleotide repeat associated with amyotrophic lateral sclerosis and frontotemporal dementia forms a triplex-like structure and binds small synthetic ligand.
Blaszczyk, L., Ryczek, M., Das, B., Mateja-Pluta, M., Bejger, M., Sliwiak, J., Nakatani, K., Kiliszek, A.(2024) Nucleic Acids Res 52: 6707-6717
- PubMed: 38738637
- DOI: https://doi.org/10.1093/nar/gkae376
- Primary Citation of Related Structures:
8QMH, 8QMI, 9EN6 - PubMed Abstract:
The abnormal expansion of GGGGCC/GGCCCC hexanucleotide repeats (HR) in C9orf72 is associated with amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Structural polymorphisms of HR result in the multifactorial pathomechanism of ALS/FTD. Consequently, many ongoing studies are focused at developing therapies targeting pathogenic HR RNA. One of them involves small molecules blocking sequestration of important proteins, preventing formation of toxic nuclear foci. However, rational design of potential therapeutics is hindered by limited number of structural studies of RNA-ligand complexes. We determined the crystal structure of antisense HR RNA in complex with ANP77 ligand (1.1 Å resolution) and in the free form (0.92 and 1.5 Å resolution). HR RNA folds into a triplex structure composed of four RNA chains. ANP77 interacted with two neighboring single-stranded cytosines to form pseudo-canonical base pairs by adopting sandwich-like conformation and adjusting the position of its naphthyridine units to the helical twist of the RNA. In the unliganded structure, the cytosines formed a peculiar triplex i-motif, assembled by trans C•C+ pair and a third cytosine located at the Hoogsteen edge of the C•C+ pair. These results extend our knowledge of the structural polymorphisms of HR and can be used for rational design of small molecules targeting disease-related RNAs.
Organizational Affiliation:
Institute of Bioorganic Chemistry, Polish Academy of Sciences, Z. Noskowskiego 12/14, 61-704, Poland.