compound 1a bound KMT9 crystal structure
Sheng, W., Eric, M., Roland, S.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Methyltransferase N6AMT1 | 203 | Homo sapiens | Mutation(s): 0 Gene Names: N6AMT1, C21orf127, HEMK2, KMT9, PRED28 EC: 2.1.1 | 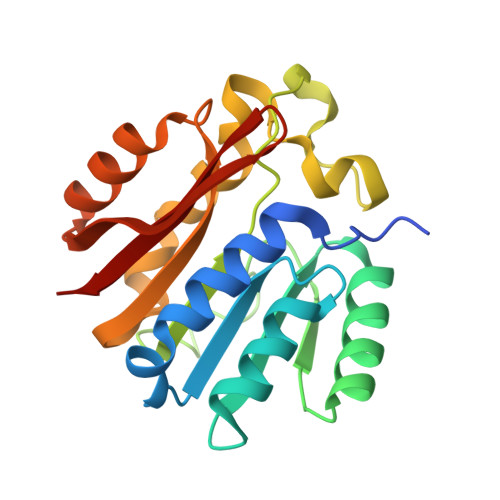 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9Y5N5 (Homo sapiens) Explore Q9Y5N5 Go to UniProtKB: Q9Y5N5 | |||||
PHAROS: Q9Y5N5 GTEx: ENSG00000156239 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9Y5N5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Multifunctional methyltransferase subunit TRM112-like protein | 126 | Homo sapiens | Mutation(s): 0 Gene Names: TRMT112, AD-001, HSPC152, HSPC170 | 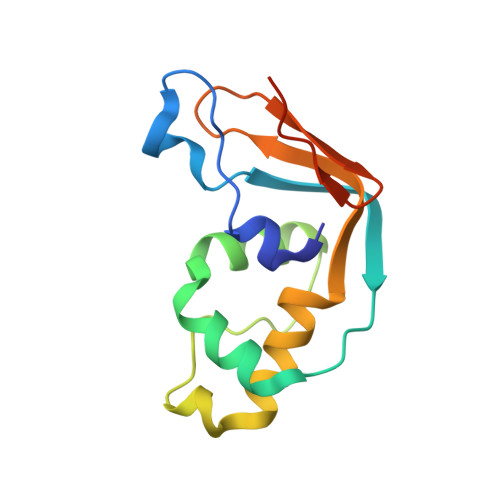 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9UI30 (Homo sapiens) Explore Q9UI30 Go to UniProtKB: Q9UI30 | |||||
PHAROS: Q9UI30 GTEx: ENSG00000173113 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9UI30 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| QII (Subject of Investigation/LOI) Query on QII | C [auth A] | (2~{S})-4-[[(2~{R},3~{S},4~{R},5~{R})-5-(6-aminopurin-9-yl)-3,4-bis(oxidanyl)oxolan-2-yl]methyl-[2-[(2~{R})-pyrrolidin-2-yl]ethyl]amino]-2-azanyl-butanoic acid C20 H32 N8 O5 HVEHZOYFHBQABO-DAEWCUBLSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 110.516 | α = 90 |
| b = 110.516 | β = 90 |
| c = 129.837 | γ = 120 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| Aimless | data scaling |
| XDS | data reduction |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| German Research Foundation (DFG) | Germany | SFB1381 |
| German Research Foundation (DFG) | Germany | SFB992 |
| German Research Foundation (DFG) | Germany | Schu688 |
| German Research Foundation (DFG) | Germany | EXC-2189 |
| German Research Foundation (DFG) | Germany | DKTK FR01-374 |