Characterisation of RNA guanine-7 methyltransferase (RNMT) using a small molecule approach.
Pearson, L.A., Petit, A.P., Mendoza Martinez, C., Bellany, F., Lin, D., Niven, S., Swift, R., Eadsforth, T., Fyfe, P., Paul, M., Postis, V., Hu, X., Cowling, V.H., Gray, D.W.(2025) Biochem J 482
- PubMed: 39869500
- DOI: https://doi.org/10.1042/BCJ20240608
- Primary Citation of Related Structures:
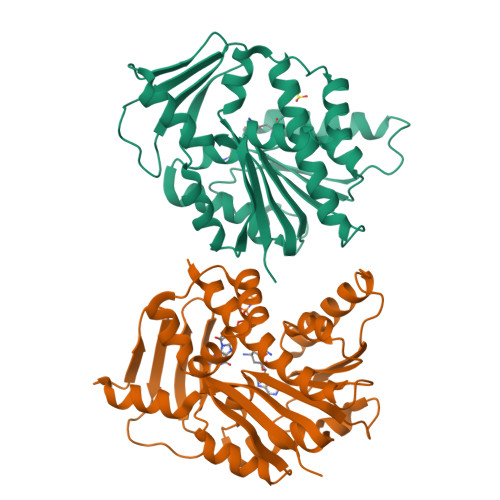
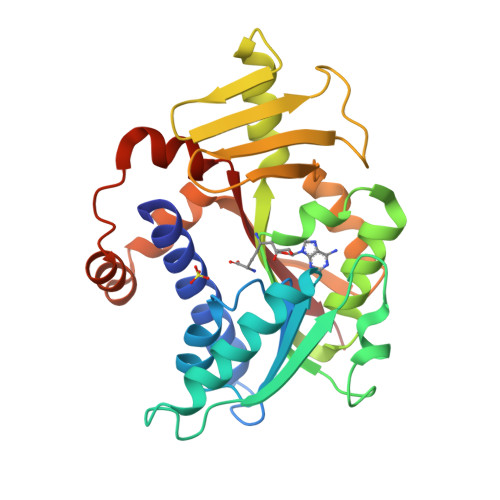
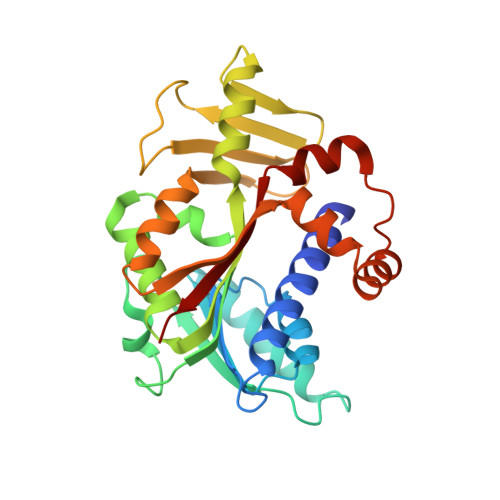
8Q69, 8Q8G, 8Q9W - PubMed Abstract:
The maturation of the RNA cap involving guanosine N-7 methylation, catalyzsed by the HsRNMT (RNA guanine-7 methyltransferase (HsRNMT)-RAM (RNA guanine-N7 methyltransferase activating subunit (RAM) complex, is currently under investigation as a novel strategy to combat PIK3CA -mutant breast cancer. However, the development of effective drugs is hindered by a limited understanding of the enzyme's mechanism and a lack of small molecule inhibitors. Following the elucidation of the HsRNMT-RAM molecular mechanism, we report the biophysical characterizsation of two small molecule hits. Biophysics, biochemistry and structural biology confirm that both compounds bind competitively with cap and bind effectively to HsRNMT-RAM in the presence of the co-product SAH, with a binding affinity (KD) of approximately 1 μM. This stabilisation of the enzyme--product complex results in uncompetitive inhibition. Finally, we describe the properties of the cap pocket and provided suggestions for further development of the tool compounds.
Organizational Affiliation:
Drug Discovery Unit, School of Life Sciences, University of Dundee, Dundee, U.K.