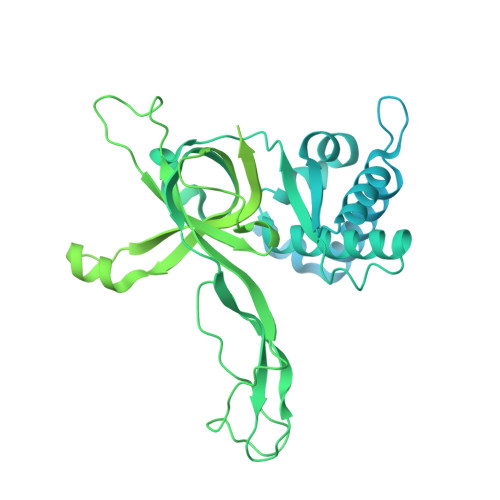
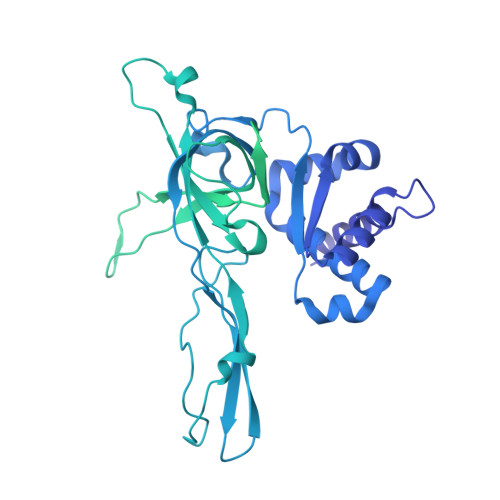
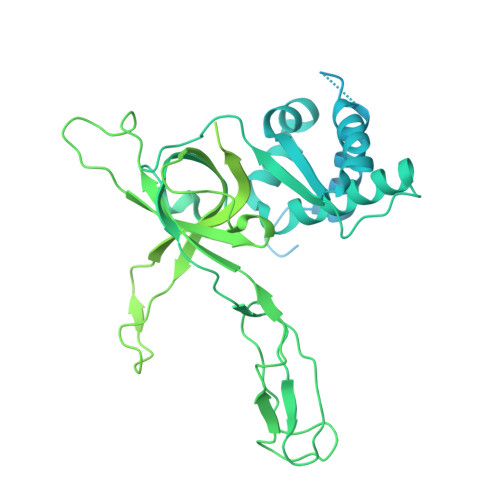
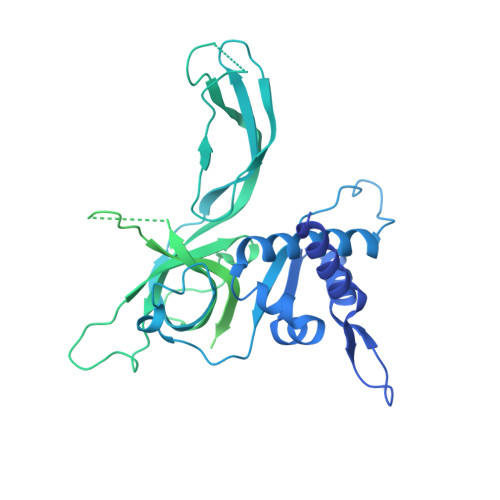
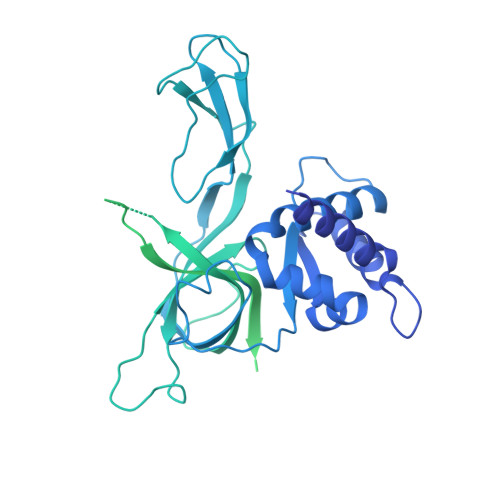
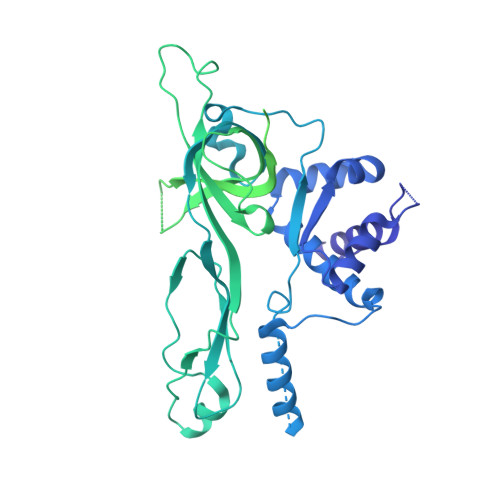
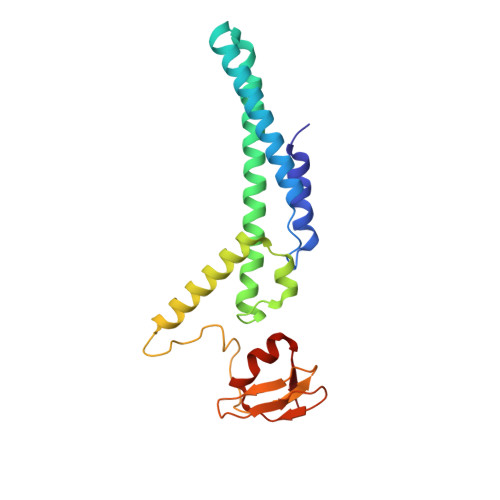
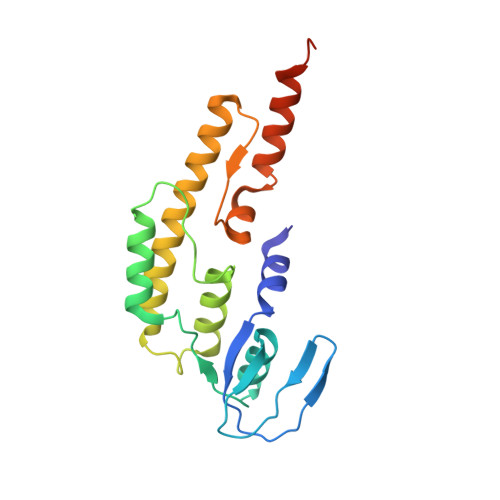
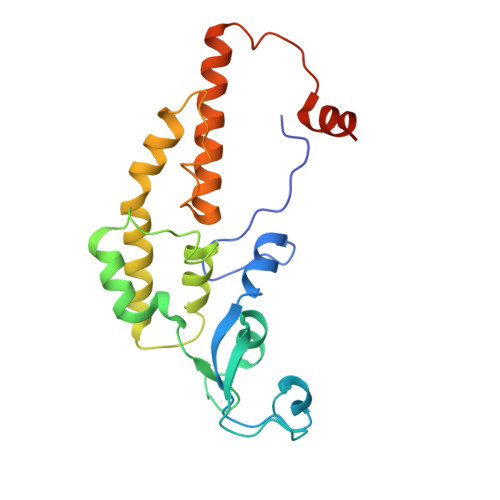
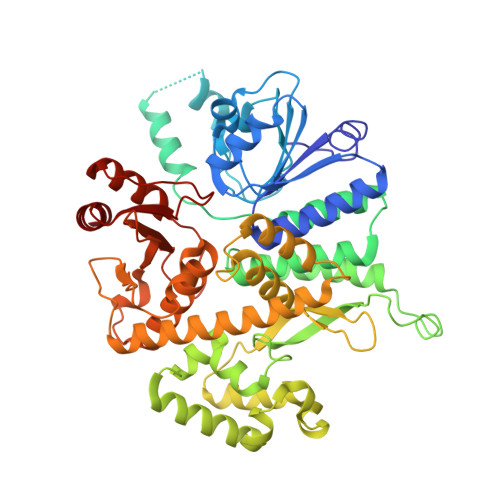
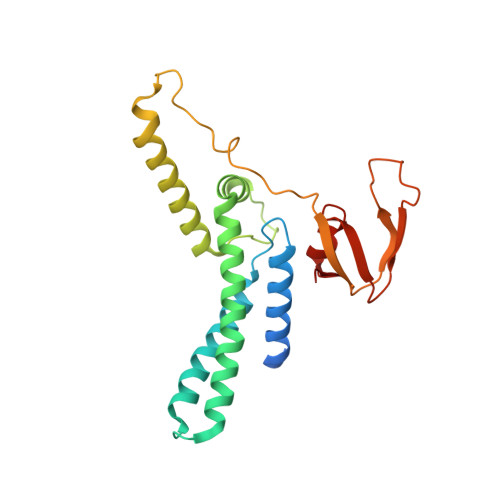
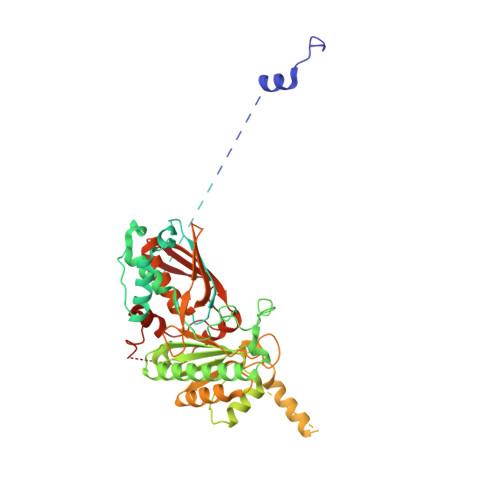
The structural mechanism of dimeric DONSON in replicative helicase activation.
Cvetkovic, M.A., Passaretti, P., Butryn, A., Reynolds-Winczura, A., Kingsley, G., Skagia, A., Fernandez-Cuesta, C., Poovathumkadavil, D., George, R., Chauhan, A.S., Jhujh, S.S., Stewart, G.S., Gambus, A., Costa, A.(2023) Mol Cell 83: 4017
- PubMed: 37820732
- DOI: https://doi.org/10.1016/j.molcel.2023.09.029
- Primary Citation of Related Structures:
8Q6O, 8Q6P - PubMed Abstract:
The MCM motor of the replicative helicase is loaded onto origin DNA as an inactive double hexamer before replication initiation. Recruitment of activators GINS and Cdc45 upon S-phase transition promotes the assembly of two active CMG helicases. Although work with yeast established the mechanism for origin activation, how CMG is formed in higher eukaryotes is poorly understood. Metazoan Downstream neighbor of Son (DONSON) has recently been shown to deliver GINS to MCM during CMG assembly. What impact this has on the MCM double hexamer is unknown. Here, we used cryoelectron microscopy (cryo-EM) on proteins isolated from replicating Xenopus egg extracts to identify a double CMG complex bridged by a DONSON dimer. We find that tethering elements mediating complex formation are essential for replication. DONSON reconfigures the MCM motors in the double CMG, and primordial dwarfism patients' mutations disrupting DONSON dimerization affect GINS and MCM engagement in human cells and DNA synthesis in Xenopus egg extracts.
Organizational Affiliation:
Macromolecular Machines Laboratory, The Francis Crick Institute, London NW1 1AT, UK.