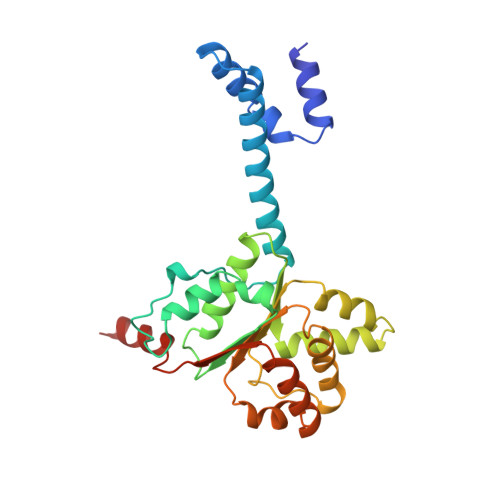
Molecular basis for transposase activation by a dedicated AAA+ ATPase.
de la Gandara, A., Spinola-Amilibia, M., Araujo-Bazan, L., Nunez-Ramirez, R., Berger, J.M., Arias-Palomo, E.(2024) Nature 630: 1003-1011
- PubMed: 38926614
- DOI: https://doi.org/10.1038/s41586-024-07550-6
- Primary Citation of Related Structures:
8Q3W, 8Q4D - PubMed Abstract:
Transposases drive chromosomal rearrangements and the dissemination of drug-resistance genes and toxins 1-3 . Although some transposases act alone, many rely on dedicated AAA+ ATPase subunits that regulate site selectivity and catalytic function through poorly understood mechanisms. Using IS21 as a model transposase system, we show how an ATPase regulator uses nucleotide-controlled assembly and DNA deformation to enable structure-based site selectivity, transposase recruitment, and activation and integration. Solution and cryogenic electron microscopy studies show that the IstB ATPase self-assembles into an autoinhibited pentamer of dimers that tightly curves target DNA into a half-coil. Two of these decamers dimerize, which stabilizes the target nucleic acid into a kinked S-shaped configuration that engages the IstA transposase at the interface between the two IstB oligomers to form an approximately 1 MDa transpososome complex. Specific interactions stimulate regulator ATPase activity and trigger a large conformational change on the transposase that positions the catalytic site to perform DNA strand transfer. These studies help explain how AAA+ ATPase regulators-which are used by classical transposition systems such as Tn7, Mu and CRISPR-associated elements-can remodel their substrate DNA and cognate transposases to promote function.
Organizational Affiliation:
Centro de Investigaciones Biológicas Margarita Salas, CSIC, Madrid, Spain.