Structural and mechanistic basis of the central energy-converting methyltransferase complex of methanogenesis.
Aziz, I., Kayastha, K., Kaltwasser, S., Vonck, J., Welsch, S., Murphy, B.J., Kahnt, J., Wu, D., Wagner, T., Shima, S., Ermler, U.(2024) Proc Natl Acad Sci U S A 121: e2315568121-e2315568121
- PubMed: 38530900
- DOI: https://doi.org/10.1073/pnas.2315568121
- Primary Citation of Related Structures:
8Q3V - PubMed Abstract:
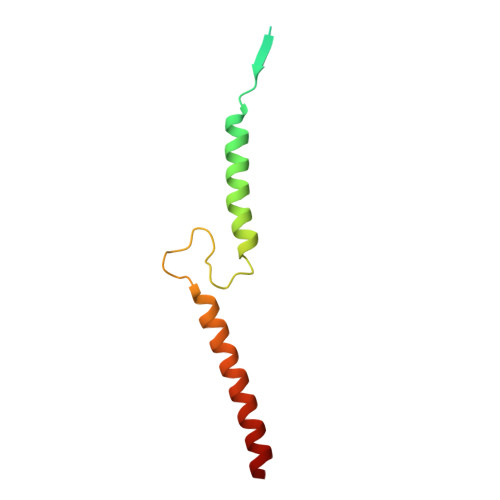
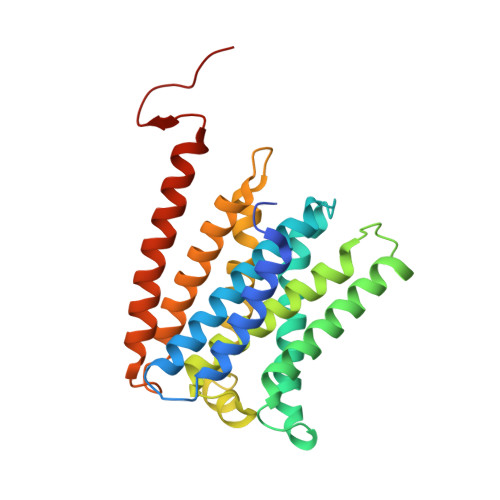
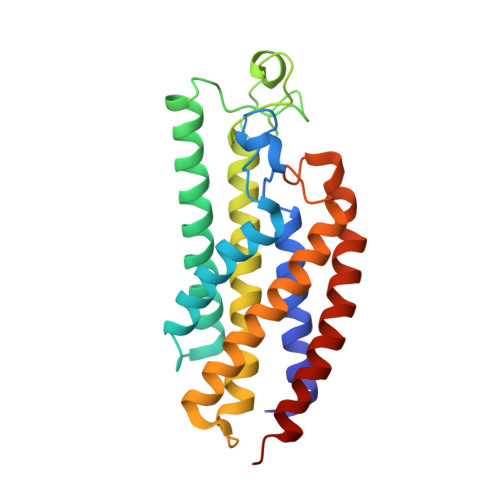
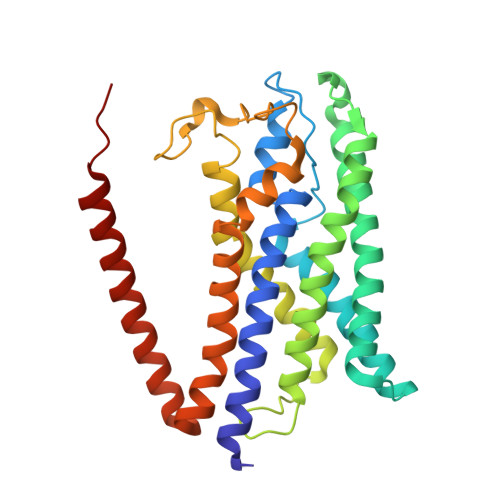
Methanogenic archaea inhabiting anaerobic environments play a crucial role in the global biogeochemical material cycle. The most universal electrogenic reaction of their methane-producing energy metabolism is catalyzed by N 5 -methyl-tetrahydromethanopterin: coenzyme M methyltransferase (MtrABCDEFGH), which couples the vectorial Na + transport with a methyl transfer between the one-carbon carriers tetrahydromethanopterin and coenzyme M via a vitamin B 12 derivative (cobamide) as prosthetic group. We present the 2.08 Å cryo-EM structure of Mtr(ABCDEFG) 3 composed of the central Mtr(ABFG) 3 stalk symmetrically flanked by three membrane-spanning MtrCDE globes. Tetraether glycolipids visible in the map fill gaps inside the multisubunit complex. Putative coenzyme M and Na + were identified inside or in a side-pocket of a cytoplasmic cavity formed within MtrCDE. Its bottom marks the gate of the transmembrane pore occluded in the cryo-EM map. By integrating Alphafold2 information, functionally competent MtrA-MtrH and MtrA-MtrCDE subcomplexes could be modeled and thus the methyl-tetrahydromethanopterin demethylation and coenzyme M methylation half-reactions structurally described. Methyl-transfer-driven Na + transport is proposed to be based on a strong and weak complex between MtrCDE and MtrA carrying vitamin B 12 , the latter being placed at the entrance of the cytoplasmic MtrCDE cavity. Hypothetically, strongly attached methyl-cob(III)amide (His-on) carrying MtrA induces an inward-facing conformation, Na + flux into the membrane protein center and finally coenzyme M methylation while the generated loosely attached (or detached) MtrA carrying cob(I)amide (His-off) induces an outward-facing conformation and an extracellular Na + outflux. Methyl-cob(III)amide (His-on) is regenerated in the distant active site of the methyl-tetrahydromethanopterin binding MtrH implicating a large-scale shuttling movement of the vitamin B 12 -carrying domain.
Organizational Affiliation:
Molecular Membrane Biology, Max Planck Institute of Biophysics, Frankfurt am Main D-60438, Germany.