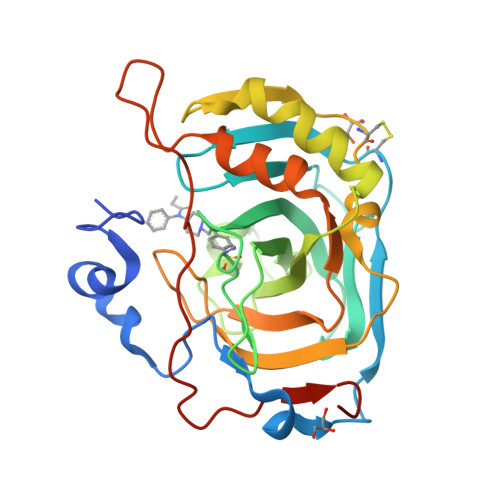
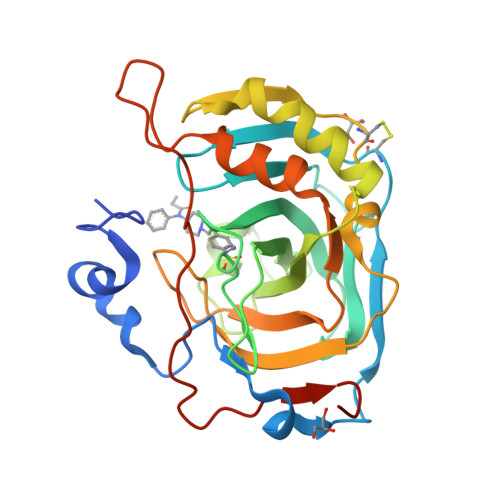
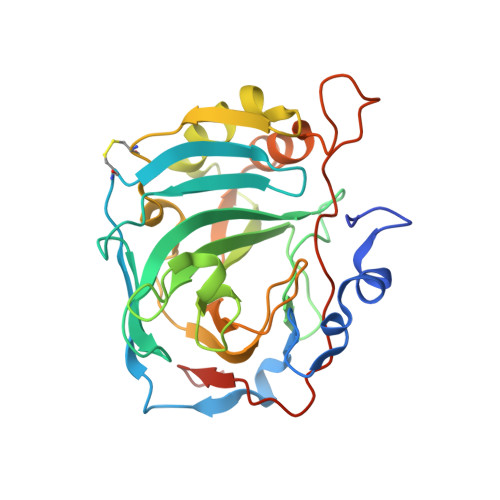
Discovery of a novel series of potent carbonic anhydrase inhibitors with selective affinity for mu Opioid receptor for Safer and long-lasting analgesia.
Angeli, A., Micheli, L., Turnaturi, R., Pasquinucci, L., Parenti, C., Alterio, V., Di Fiore, A., De Simone, G., Monti, S.M., Carta, F., Di Cesare Mannelli, L., Ghelardini, C., Supuran, C.T.(2023) Eur J Med Chem 260: 115783-115783
- PubMed: 37678143
- DOI: https://doi.org/10.1016/j.ejmech.2023.115783
- Primary Citation of Related Structures:
8Q3U - PubMed Abstract:
In this study, we investigated the development of dual-targeted ligands that bind to both μ-opioid receptor (MOR) and carbonic anhydrase (CA) enzymes, using fentanyl structure as a template. We synthesized and evaluated 21 novel compounds with dual-targeted affinity identifying the lead candidate compound 8, showing selective affinity for MOR and potent inhibition of several cytosolic CA isoforms. By means of repeated treatment of 3 daily administrations for 17 days, fentanyl (0.1 mg/kg, subcutaneously) led to tolerance development, pain threshold alterations and withdrawal symptoms in CD-1 mice, as well as astrocyte and microglia activation in the dorsal horn of the lumbar spinal cord. In contrast, compound 8 (0.32 mg/kg s.c.) maintained stable during days its analgesic effect at the higher dose tested with fewer withdrawal symptoms, allodynia development and glial cells activation. Our results suggest that targeting both MOR and CA enzymes can lead to the development of new class of potent analgesic agents with fewer side effects and reduced tolerance development. Further studies are needed to explore the potential mechanisms underlying these effects and to further optimize the therapeutic potential of these compounds.
Organizational Affiliation:
NEUROFARBA Department, Sezione di Scienze Farmaceutiche, University of Florence, Via Ugo Schiff 6, 50019, Sesto Fiorentino, Florence, Italy. Electronic address: andrea.angeli@unifi.it.