Active Pcd complex with prFMN bound
Kayastha, K., Ermler, U.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Phthaloyl-CoA decarboxylase | 527 | Thauera chlorobenzoica | Mutation(s): 0 Gene Names: Tchl_3080 | 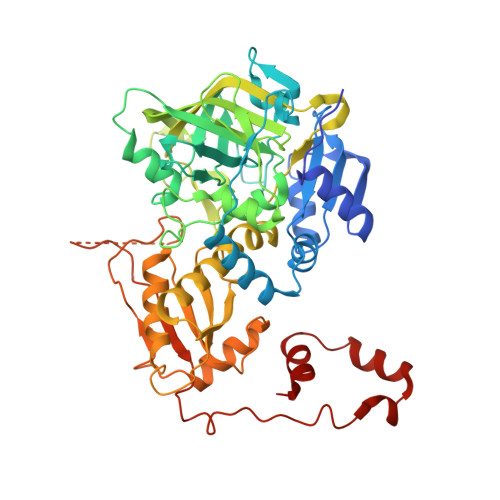 | |
UniProt | |||||
Find proteins for A0A193DUB4 (Thauera chlorobenzoica) Explore A0A193DUB4 Go to UniProtKB: A0A193DUB4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A193DUB4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| BYN (Subject of Investigation/LOI) Query on BYN | AA [auth E] FA [auth F] G [auth A] L [auth B] Q [auth C] | hydroxylated prenyl-FMN C22 H31 N4 O10 P BDNVCRCOZIAGID-LWGWVAHUSA-N |  | ||
| FE (Subject of Investigation/LOI) Query on FE | BA [auth E] CA [auth E] DA [auth E] GA [auth F] H [auth A] | FE (III) ION Fe VTLYFUHAOXGGBS-UHFFFAOYSA-N |  | ||
| K (Subject of Investigation/LOI) Query on K | EA [auth E] JA [auth F] K [auth A] P [auth B] U [auth C] | POTASSIUM ION K NPYPAHLBTDXSSS-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | RELION | 3.1 |
| MODEL REFINEMENT | PHENIX | 1.14 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Max Planck Society | Germany | -- |