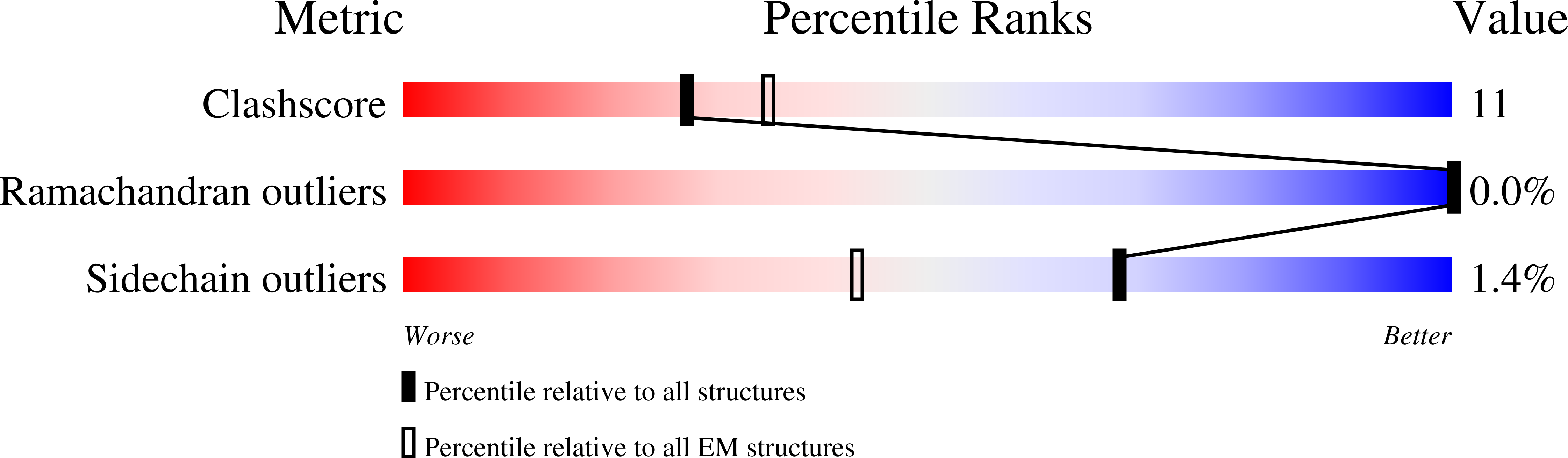Cryo-electron tomography reveals the binding and release states of the major adhesion complex from Mycoplasma genitalium.
Sprankel, L., Scheffer, M.P., Manger, S., Ermel, U.H., Frangakis, A.S.(2023) PLoS Pathog 19: e1011761-e1011761
- PubMed: 37939157
- DOI: https://doi.org/10.1371/journal.ppat.1011761
- Primary Citation of Related Structures:
8PBX, 8PBY, 8PBZ, 8PC0, 8PC1 - PubMed Abstract:
The nap particle is an immunogenic surface adhesion complex from Mycoplasma genitalium. It is essential for motility and responsible for binding sialylated oligosaccharides on the surface of the host cell. The nap particle is composed of two P140-P110 heterodimers, the structure of which was recently solved. However, the interpretation of the mechanism by which the mycoplasma cells orchestrate adhesion remained challenging. Here, we provide cryo-electron tomography structures at ~11 Å resolution, which allow for the distinction between the bound and released state of the nap particle, displaying the in vivo conformational states. Fitting of the atomically resolved structures reveals that bound sialylated oligosaccharides are stabilized by both P110 and P140. Movement of the stalk domains allows for the transfer of conformational changes from the interior of the cell to the binding pocket, thus having the capability of an active release process. It is likely that the same mechanism can be transferred to other Mycoplasma species that belong to the pneumoniae cluster.
Organizational Affiliation:
Buchmann Institute for Molecular Life Sciences and Institute of Biophysics, Goethe University Frankfurt, Frankfurt, Germany.