Beyond genetics: Deciphering the impact of missense variants in CAD deficiency.
Del Cano-Ochoa, F., Ng, B.G., Rubio-Del-Campo, A., Mahajan, S., Wilson, M.P., Vilar, M., Rymen, D., Sanchez-Pintos, P., Kenny, J., Ley Martos, M., Campos, T., Wortmann, S.B., Freeze, H.H., Ramon-Maiques, S.(2023) J Inherit Metab Dis 46: 1170-1185
- PubMed: 37540500
- DOI: https://doi.org/10.1002/jimd.12667
- Primary Citation of Related Structures:
8PBE, 8PBG, 8PBH, 8PBI, 8PBJ, 8PBK, 8PBM, 8PBN, 8PBP, 8PBQ, 8PBR, 8PBS, 8PBT, 8PBU - PubMed Abstract:
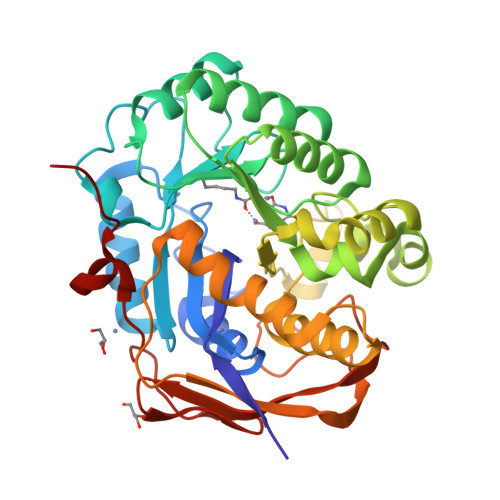
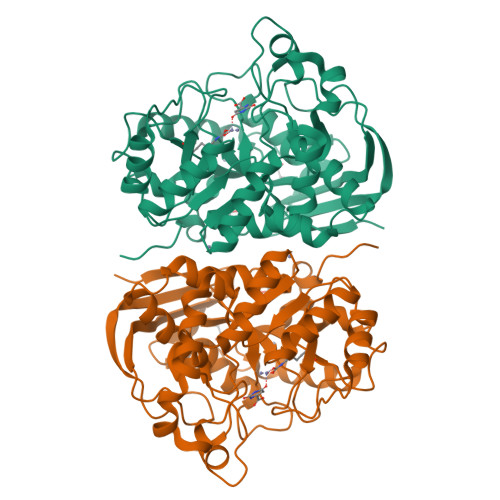
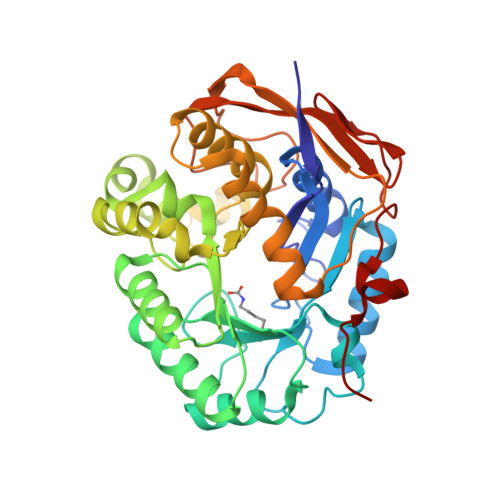
CAD is a large, 2225 amino acid multienzymatic protein required for de novo pyrimidine biosynthesis. Pathological CAD variants cause a developmental and epileptic encephalopathy which is highly responsive to uridine supplements. CAD deficiency is difficult to diagnose because symptoms are nonspecific, there is no biomarker, and the protein has over 1000 known variants. To improve diagnosis, we assessed the pathogenicity of 20 unreported missense CAD variants using a growth complementation assay that identified 11 pathogenic variants in seven affected individuals; they would benefit from uridine treatment. We also tested nine variants previously reported as pathogenic and confirmed the damaging effect of seven. However, we reclassified two variants as likely benign based on our assay, which is consistent with their long-term follow-up with uridine. We found that several computational methods are unreliable predictors of pathogenic CAD variants, so we extended the functional assay results by studying the impact of pathogenic variants at the protein level. We focused on CAD's dihydroorotase (DHO) domain because it accumulates the largest density of damaging missense changes. The atomic-resolution structures of eight DHO pathogenic variants, combined with functional and molecular dynamics analyses, provided a comprehensive structural and functional understanding of the activity, stability, and oligomerization of CAD's DHO domain. Combining our functional and protein structural analysis can help refine clinical diagnostic workflow for CAD variants in the genomics era.
Organizational Affiliation:
Structure of Macromolecular Targets Unit, Instituto de Biomedicina de Valencia (IBV), CSIC, Valencia, Spain.