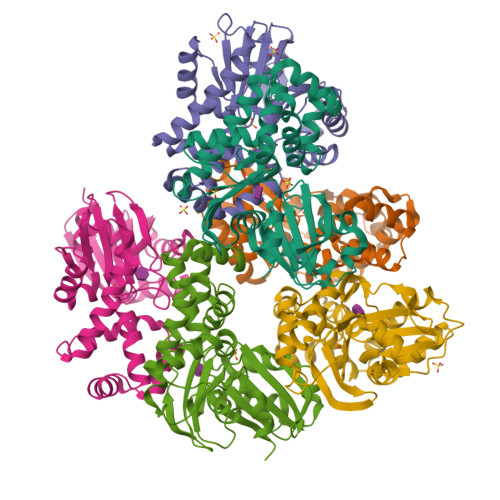
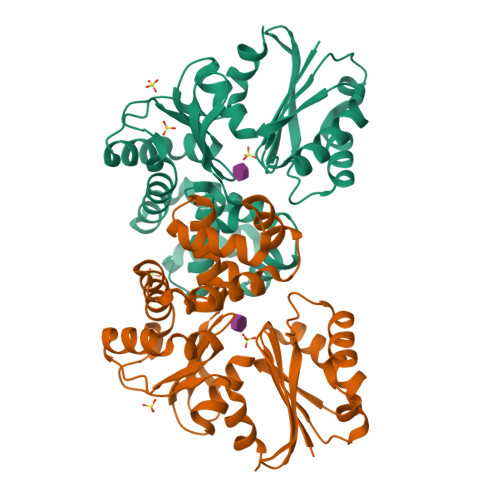
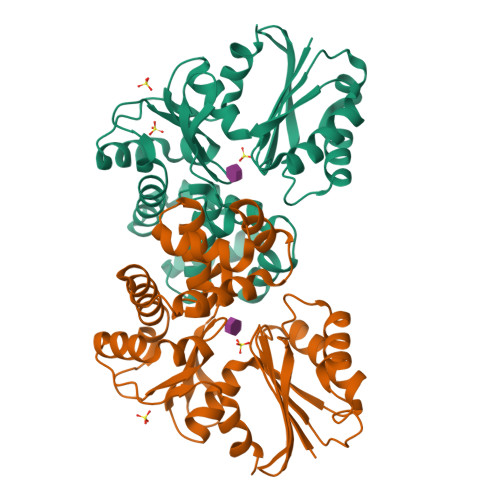
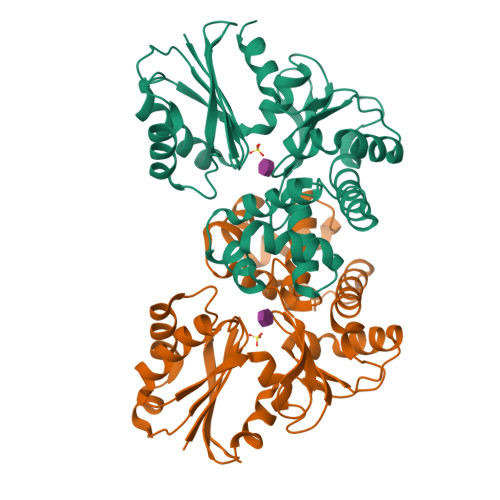
N-acetylmuramic acid recognition by MurK kinase from the MurNAc auxotrophic oral pathogen Tannerella forsythia.
Stasiak, A.C., Gogler, K., Borisova, M., Fink, P., Mayer, C., Stehle, T., Zocher, G.(2023) J Biological Chem 299: 105076-105076
- PubMed: 37481208
- DOI: https://doi.org/10.1016/j.jbc.2023.105076
- Primary Citation of Related Structures:
8OQK, 8OQW, 8OQX, 8OW7, 8OW9 - PubMed Abstract:
The bacterial cell wall consists of a three-dimensional peptidoglycan layer, composed of peptides linked to the sugars N-acetylmuramic acid (MurNAc) and GlcNAc. Unlike other bacteria, the pathogenic Tannerella forsythia, a member of the red complex group of bacteria associated with the late stages of periodontitis, lacks biosynthetic pathways for MurNAc production and therefore obtains MurNAc from the environment. Sugar kinases play a crucial role in the MurNAc recycling process, activating the sugar molecules by phosphorylation. In this study, we present the first crystal structures of a MurNAc kinase, called murein sugar kinase (MurK), in its unbound state as well as in complexes with the ATP analog β-γ-methylene adenosine triphosphate (AMP-PCP) and with MurNAc. We also determined the crystal structures of K1058, a paralogous MurNAc kinase of T. forsythia, in its unbound state and in complex with MurNAc. We identified the active site and residues crucial for MurNAc specificity as the less bulky side chains of S133, P134, and L135, which enlarge the binding cavity for the lactyl ether group, unlike the glutamate or histidine residues present in structural homologs. In establishing the apparent kinetic parameters for both enzymes, we showed a comparable affinity for MurNAc (K m 180 μM and 30 μM for MurK and K1058, respectively), with MurK being over two hundred times faster than K1058 (V max 80 and 0.34 μmol min -1 mg -1 , respectively). These data might support a structure-guided approach to development of inhibitory MurNAc analogs for pathogen MurK enzymes.
Organizational Affiliation:
Interfaculty Institute of Biochemistry, University of Tuebingen, Tuebingen, Germany.