Leveraging Ligand Affinity and Properties: Discovery of Novel Benzamide-Type Cereblon Binders for the Design of PROTACs.
Steinebach, C., Bricelj, A., Murgai, A., Sosic, I., Bischof, L., Ng, Y.L.D., Heim, C., Maiwald, S., Proj, M., Voget, R., Feller, F., Kosmrlj, J., Sapozhnikova, V., Schmidt, A., Zuleeg, M.R., Lemnitzer, P., Mertins, P., Hansen, F.K., Gutschow, M., Kronke, J., Hartmann, M.D.(2023) J Med Chem 66: 14513-14543
- PubMed: 37902300
- DOI: https://doi.org/10.1021/acs.jmedchem.3c00851
- Primary Citation of Related Structures:
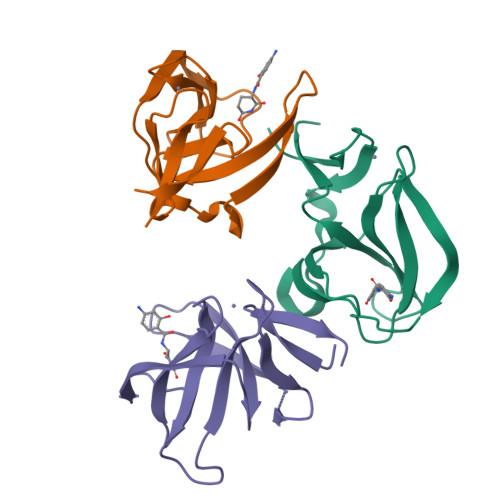
8OU3, 8OU4, 8OU5, 8OU6, 8OU7, 8OU9, 8OUA - PubMed Abstract:
Immunomodulatory imide drugs (IMiDs) such as thalidomide, pomalidomide, and lenalidomide are the most common cereblon (CRBN) recruiters in proteolysis-targeting chimera (PROTAC) design. However, these CRBN ligands induce the degradation of IMiD neosubstrates and are inherently unstable, degrading hydrolytically under moderate conditions. In this work, we simultaneously optimized physiochemical properties, stability, on-target affinity, and off-target neosubstrate modulation features to develop novel nonphthalimide CRBN binders. These efforts led to the discovery of conformationally locked benzamide-type derivatives that replicate the interactions of the natural CRBN degron, exhibit enhanced chemical stability, and display a favorable selectivity profile in terms of neosubstrate recruitment. The utility of the most potent ligands was demonstrated by their transformation into potent degraders of BRD4 and HDAC6 that outperform previously described reference PROTACs. Together with their significantly decreased neomorphic ligase activity on IKZF1/3 and SALL4, these ligands provide opportunities for the design of highly selective and potent chemically inert proximity-inducing compounds.
Organizational Affiliation:
Pharmaceutical Institute, University of Bonn, D-53121 Bonn, Germany.