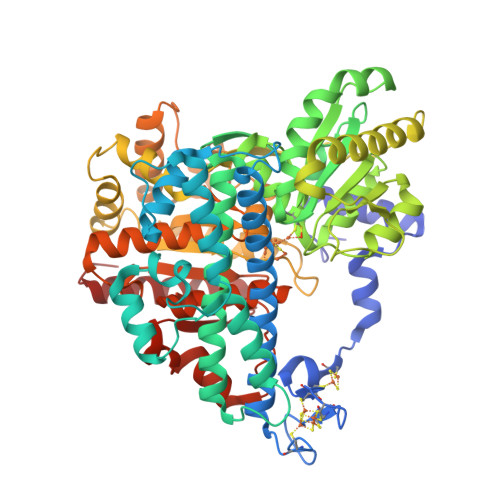
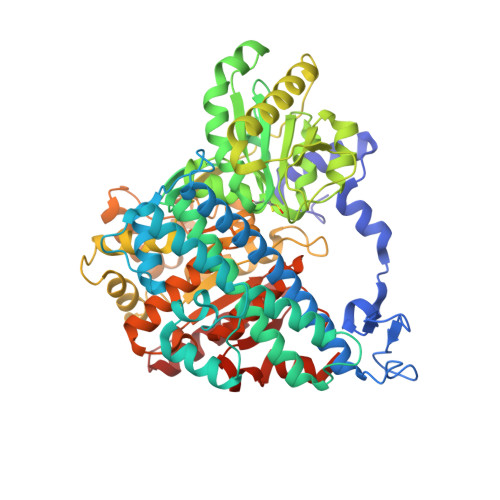
Stepwise O 2 -Induced Rearrangement and Disassembly of the [NiFe 4 (OH)( mu 3 -S) 4 ] Active Site Cluster of CO Dehydrogenase.
Basak, Y., Jeoung, J.H., Domnik, L., Dobbek, H.(2023) Angew Chem Int Ed Engl 62: e202305341-e202305341
- PubMed: 37279092
- DOI: https://doi.org/10.1002/anie.202305341
- Primary Citation of Related Structures:
8OMX, 8OMY, 8ON0, 8ON1, 8ON2, 8ON3 - PubMed Abstract:
Ni,Fe-containing carbon monoxide dehydrogenases (CODHs) catalyze the reversible reduction of carbon dioxide to carbon monoxide. CODHs are found in anaerobic microorganisms and can rapidly lose their activity when exposed to air. What causes the loss of activity is unclear. In this study, we analyzed the time-dependent structural changes induced by the presence of air on the metal centers of CODH-II. We show that inactivation is a multistep process. In a reversible step, the open coordination site on the Ni ion is blocked by a Ni,Fe-bridging μ-sulfido or chlorido ligand. Blocking this open coordination site with a cyanide ligand stabilizes the cluster against O 2 -induced decomposition, indicating that O 2 attacks at the Ni ion. In the subsequent irreversible phase, nickel is lost, the Fe ions rearrange and the sulfido ligands disappear. Our data are consistent with a reversible reductive reactivation mechanism to protect CODHs from transient over-oxidation.
Organizational Affiliation:
Institut für Biologie, Humboldt-Universität zu Berlin, Unter den Linden 6, 10099, Berlin, Germany.