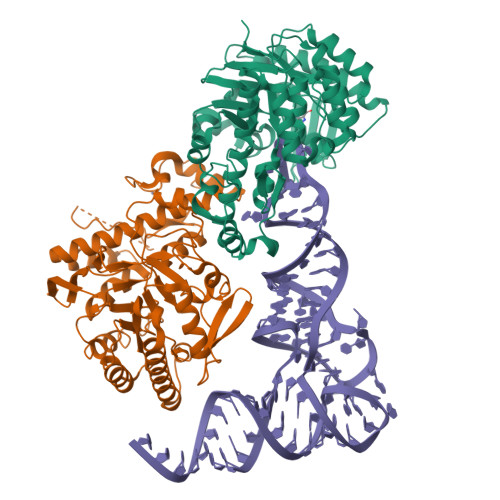
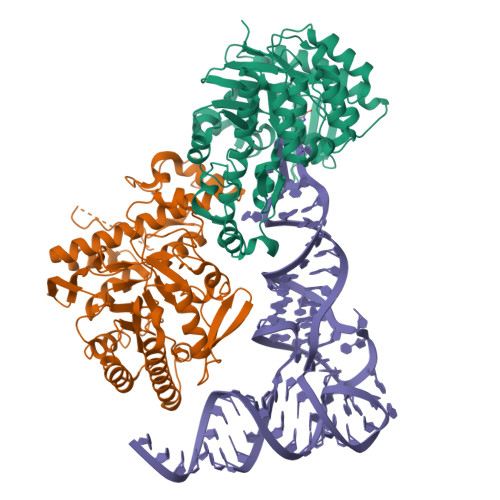
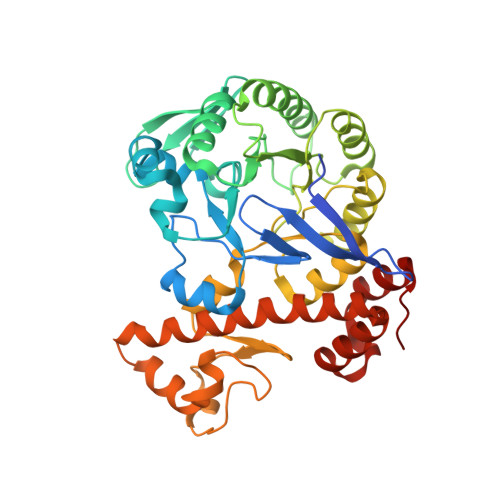
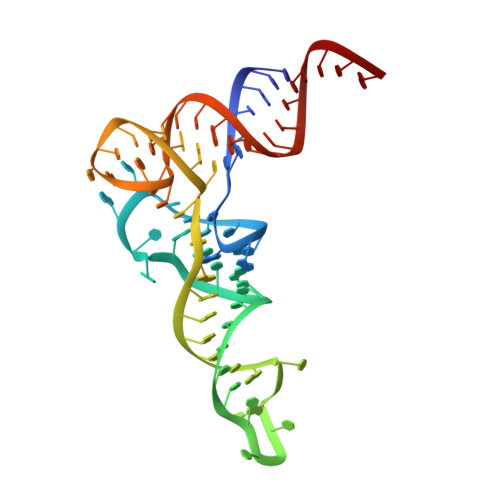
Structural and functional insights into tRNA recognition by human tRNA guanine transglycosylase.
Sievers, K., Neumann, P., Susac, L., Da Vela, S., Graewert, M., Trowitzsch, S., Svergun, D., Tampe, R., Ficner, R.(2024) Structure 32: 316
- PubMed: 38181786
- DOI: https://doi.org/10.1016/j.str.2023.12.006
- Primary Citation of Related Structures:
8OMR - PubMed Abstract:
Eukaryotic tRNA guanine transglycosylase (TGT) is an RNA-modifying enzyme which catalyzes the base exchange of the genetically encoded guanine 34 of tRNAs Asp,Asn,His,Tyr for queuine, a hypermodified 7-deazaguanine derivative. Eukaryotic TGT is a heterodimer comprised of a catalytic and a non-catalytic subunit. While binding of the tRNA anticodon loop to the active site is structurally well understood, the contribution of the non-catalytic subunit to tRNA binding remained enigmatic, as no complex structure with a complete tRNA was available. Here, we report a cryo-EM structure of eukaryotic TGT in complex with a complete tRNA, revealing the crucial role of the non-catalytic subunit in tRNA binding. We decipher the functional significance of these additional tRNA-binding sites, analyze solution state conformation, flexibility, and disorder of apo TGT, and examine conformational transitions upon tRNA binding.
Organizational Affiliation:
Department of Molecular Structural Biology, GZMB, University of Göttingen, 37077 Göttingen, Germany.