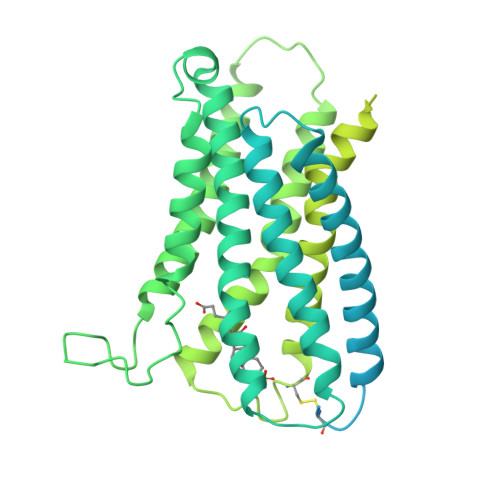
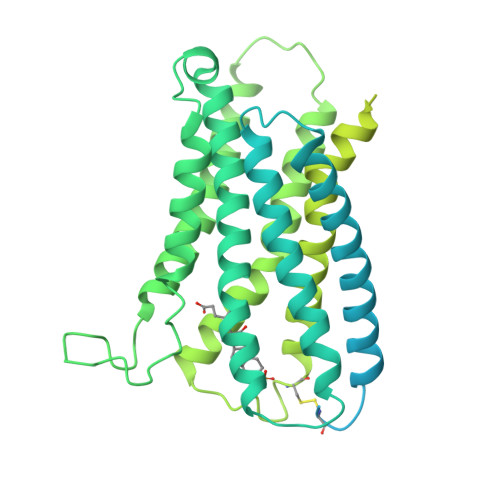
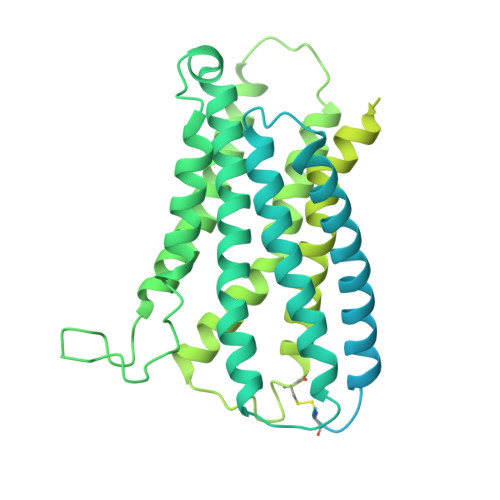
Structure-guided discovery of bile acid derivatives for treating liver diseases without causing itch.
Yang, J., Zhao, T., Fan, J., Zou, H., Lan, G., Guo, F., Shi, Y., Ke, H., Yu, H., Yue, Z., Wang, X., Bai, Y., Li, S., Liu, Y., Wang, X., Chen, Y., Li, Y., Lei, X.(2024) Cell 187: 7164-7182.e18
- PubMed: 39476841
- DOI: https://doi.org/10.1016/j.cell.2024.10.001
- Primary Citation of Related Structures:
8K4S, 8KEX - PubMed Abstract:
Chronic itch is a debilitating symptom profoundly impacting the quality of life in patients with liver diseases like cholestasis. Activation of the human G-protein coupled receptor, MRGPRX4 (hX4), by bile acids (BAs) is implicated in promoting cholestasis itch. However, the detailed underlying mechanisms remain elusive. Here, we identified 3-sulfated BAs that are elevated in cholestatic patients with itch symptoms. We solved the cryo-EM structure of hX4-Gq in a complex with 3-phosphated deoxycholic acid (DCA-3P), a mimic of the endogenous 3-sulfated deoxycholic acid (DCA-3S). This structure revealed an unprecedented ligand-binding pocket in MRGPR family proteins, highlighting the crucial role of the 3-hydroxyl (3-OH) group on BAs in activating hX4. Guided by this structural information, we designed and developed compound 7 (C7), a BA derivative lacking the 3-OH. Notably, C7 effectively alleviates hepatic injury and fibrosis in liver disease models while significantly mitigating the itch side effects.
Organizational Affiliation:
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China; Peking-Tsinghua Center for Life Sciences, Academy for Advanced Interdisciplinary Studies, Peking University, Beijing 100871, China.