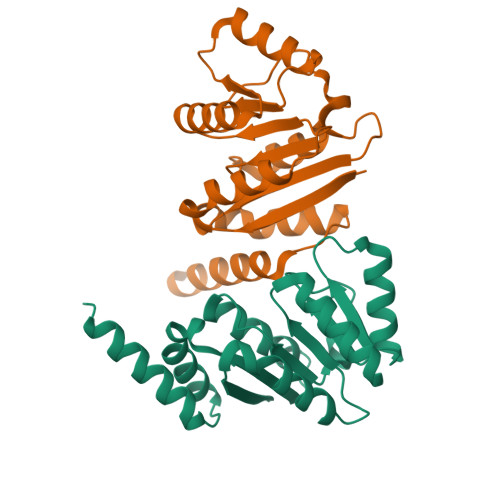
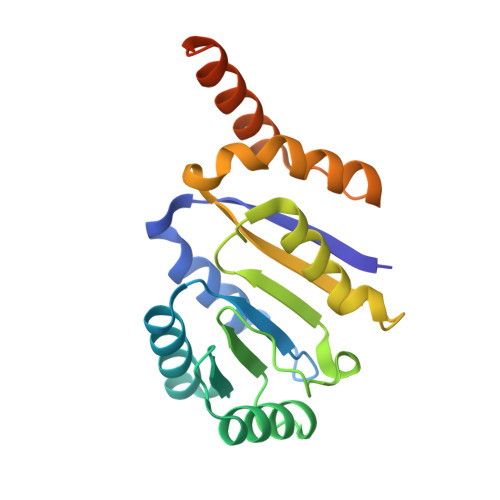
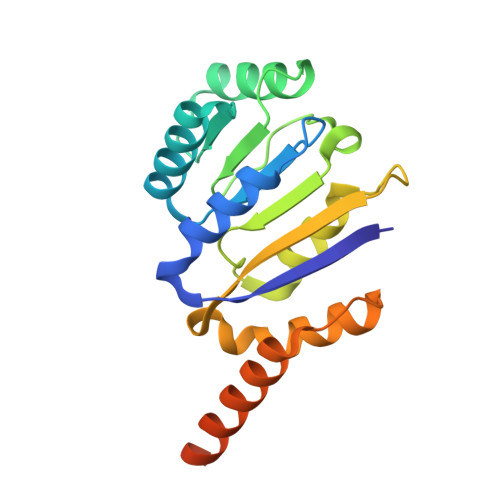
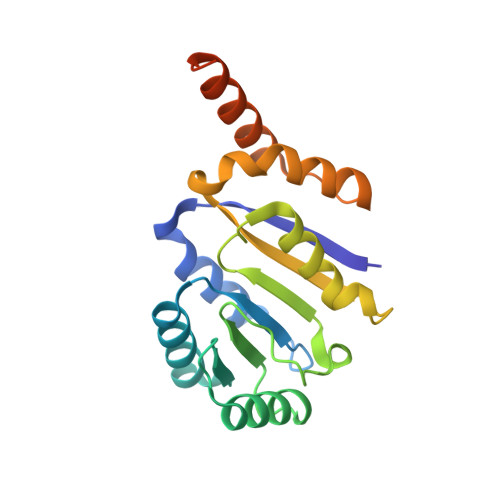
Structural insight into crystal structure of helicase domain of DDX53.
Park, S., Yang, J.B., Park, Y.H., Kim, Y.K., Jeoung, D., Kim, H.Y., Jung, H.S.(2023) Biochem Biophys Res Commun 677: 190-195
- PubMed: 37603933
- DOI: https://doi.org/10.1016/j.bbrc.2023.08.022
- Primary Citation of Related Structures:
8KCA - PubMed Abstract:
DEAD box helicase proteins are a family of RNA helicases that participate in various RNA metabolisms such as RNA unwinding, RNA processing, and RNPase activities. A particular DEAD box protein, the DDX53 protein, is primarily expressed in cancer cells and plays a crucial role in tumorigenesis. Numerous studies have revealed that DDX53 interacts with various microRNA and Histone deacetylases. However, its molecular structure and the detailed binding interaction between DDX53 and microRNA or HDAC is still unclear. In this study, we used X-ray crystallography to investigate the 3D structure of the hlicase C-terminal domain of DDX53, and successfully determined its crystal structure at a resolution of 1.97 Å. Subsequently, a functional analysis of RNA was conducted by examining the binding properties thereof with DDX53 by transmission electron microscopy and computing-based molecular docking simulation. The defined 3D model of DDX53 not only provides a structural basis for the fundamental understanding of DDX53 but is also expected to contribute to the field of anti-cancer drug discovery such as structure-based drug discovery and computer-aided drug design.
Organizational Affiliation:
Research Center for Bioconvergence Analysis, Division of Analytical Science Research, Korea Basic Science Institute, Cheongju, Chungbuk, 28119, Republic of Korea.